2019 European Thyroid Association Guidelines for the Treatment and Follow-Up of Advanced Radioiodine-Refractory Thyroid Cancer
- PMID: 31768334
- PMCID: PMC6873012
- DOI: 10.1159/000502229
2019 European Thyroid Association Guidelines for the Treatment and Follow-Up of Advanced Radioiodine-Refractory Thyroid Cancer
Abstract
The vast majority of thyroid cancers of follicular origin (TC) have a very favourable outcome, but 5-10% of cases will develop metastatic disease. Around 60-70% of this subset, hence less than 5% of all patients with TC, will become radioiodine refractory (RAI-R), with a significant negative impact on prognosis and a mean life expectancy of 3-5 years. Since no European expert consensus or guidance for this challenging condition is currently available, a task force of TC experts was nominated by the European Thyroid Association (ETA) to prepare this document based on the principles of clinical evidence. The task force started to work in September 2018 and after several revision rounds, prepared a list of recommendations to support the treatment and follow-up of patients with advanced TC. Criteria for advanced RAI-R TC were proposed, and the most appropriate diagnostic tools and the local, systemic and palliative treatments are described. Systemic therapy with multikinase inhibitors is fully discussed, including recommendations on how to start it and at which dosage, on the duration of treatment, and on the management of side effects. The appropriate relationship between the specialist and the patient/family as well as ethical issues are covered. Based on the available studies and on personal experience, the experts provided 39 recommendations aimed to improve the management of advanced RAI-R TCs. Above all of them is the indication to treat and follow these patients in a specialized setting which allows the interaction between several specialists in a multidisciplinary team.
Keywords: Advanced thyroid cancer; European Thyroid Association; Familial counselling; Lenvatinib; Local treatments; Multikinase inhibitors; Radioiodine refractory thyroid cancer; Rehabilitation; Sorafenib.
Copyright © 2019 by S. Karger AG, Basel.
Conflict of interest statement
L.F. consults for Eisai Europe Limited, Sanofi-Genzyme. R.E. consults for Eisai Europe Limited, Sanofi-Genzyme, Exilixis, and LOXO. D.F. consults for Eisai Europe Limited, Sanofi-Genzyme, Ipsen, and Novartis. B.J. is a member of AstraZeneca and Sobi Advisory Boards; has received honoraria from Amgen, Bayer Health Care, Eisai, Exelixis, Ipsen, Novartis, Oxigene, Pfizer, and Sanofi-Genzyme. S.L. is a member of Sanofi Genzyme, EISAI, Loxo, and Bayer Advisory Boards; has received research grants from Sanofi Genzyme, Novartis, and Bayer. K.N. consults for Eisai Europe Limited, Sanofi-Genzyme. J.S. has no conflicts to disclose.
Figures
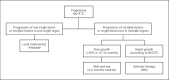
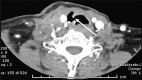

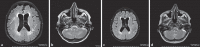
References
-
- Lloyd RV, Osamura RY, Klöppel G, Rosai J. WHO Classification of Tumours of Endocrine Organs. 4th ed. IARC WHO Classification of Tumours; 2017.
-
- Volante M, Collini P, Nikiforov YE, Sakamoto A, Kakudo K, Katoh R, et al. Poorly differentiated thyroid carcinoma: the Turin proposal for the use of uniform diagnostic criteria and an algorithmic diagnostic approach. Am J Surg Pathol. 2007 Aug;31((8)):1256–64. - PubMed
-
- Ibrahimpasic T, Ghossein R, Carlson DL, Nixon I, Palmer FL, Shaha AR, et al. Outcomes in patients with poorly differentiated thyroid carcinoma. J Clin Endocrinol Metab. 2014 Apr;99((4)):1245–52. - PubMed
-
- Baloch ZW, LiVolsi VA. Special types of thyroid carcinoma. Histopathology. 2018 Jan;72((1)):40–52. - PubMed
-
- Volante M, Bussolati G, Papotti M. The story of poorly differentiated thyroid carcinoma: from Langhans' description to the Turin proposal via Juan Rosai. Semin Diagn Pathol. 2016 Sep;33((5)):277–83. - PubMed

