Systemic Treatment for Advanced Hepatocellular Carcinoma
- PMID: 31768344
- PMCID: PMC6873089
- DOI: 10.1159/000496439
Systemic Treatment for Advanced Hepatocellular Carcinoma
Abstract
Background: Patients with advanced hepatocellular carcinoma (HCC) have a poor prognosis. First-line sorafenib has been the standard of care for a decade, but the treatment landscape is expanding. This review provides a practical overview of current and future systemic treatment options for advanced HCC and their place in clinical practice.
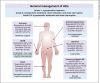
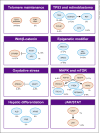
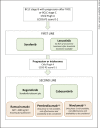
Summary: First-line sorafenib and lenvatinib have shown to improve the survival of patients with advanced HCC. In the second line, regorafenib provides benefit for patients who previously tolerated sorafenib. Anti-PD1 antibodies, nivolumab and pembrolizumab, recently became available for second-line use in the US. Ramucirumab (for patients with α-fetoprotein [AFP] levels ≥400) and cabozantinib present potential future second-line treatment options. Combinations of systemic and locoregional treatment, such as radiofrequency ablation or selective internal radiotherapy, require further research. Precision medicine has not yet been translated into clinical practice, as the most common driver mutations (TERT promoter, CTNNB1, TP53, and ARID1A mutations) have not yet been shown to be suitable therapeutic targets. However, our growing understanding of signaling pathways and efforts in drug development are expected to pave the way for precision medicine in HCC in the future. Evaluating the place for the current and novel systemic treatment options in clinical practice can be challenging due to the diverse toxicity profiles of the treatment options and characteristics of the patient population. Sorafenib data elucidate the effect patient characteristics (such as the performance score, Child-Pugh class, AFP, etiology of the underlying disease, and level of macrovascular invasion and extrahepatic spread) may have on outcomes in advanced stages.
Key messages: Lenvatinib is expected to join sorafenib as a preferred first-line treatment in advanced HCC. In the second line, the treatment of choice, regorafenib, is soon expected to be accompanied by cabozantinib and ramucirumab in patients with AFP ≥400 ng/mL, whereas nivolumab and pembrolizumab present second-line alternatives in the US.
Keywords: Hepatocellular carcinoma; Patient characteristics; Precision medicine; Targeted therapy; Treatment sequence.
Copyright © 2019 by S. Karger AG, Basel.
Conflict of interest statement
M.B. is a consultant for and has received honoraria from Bayer Pharma, Bristol-Myers Squibb, Sitex Medical. A.R.H. is a consultant for and has received honoraria from Bayer Pharma, Bristol-Myers Squibb, Merck. N.M., E.I.C., and J.-C.N. have declared no potential conflicts of interest.
Figures
References
-
- Ervik M, Lam F, Ferlay J, et al. Cancer Today. Lyon, France: International Agency for Research on Cancer Cancer Today; 2016. Available from http://gco.iarc.fr/today.
-
- Galle PR, Forner A, Llovet JM, Mazzaferro V, Piscaglia F, Raoul JL, et al. European Association for the Study of the Liver. Electronic address: easloffice@easloffice.eu. European Association for the Study of the Liver EASL clinical practice guidelines: management of hepatocellular carcinoma. J Hepatol. 2018 Jul;69((1)):182–236. - PubMed
-
- Forner A, Reig M, Bruix J. Hepatocellular carcinoma. Lancet. 2018 Mar;391((10127)):1301–14. - PubMed
-
- Llovet JM, Ricci S, Mazzaferro V, Hilgard P, Gane E, Blanc JF, et al. SHARP Investigators Study Group Sorafenib in advanced hepatocellular carcinoma. N Engl J Med. 2008 Jul;359((4)):378–90. - PubMed
-
- Bruix J, Raoul JL, Sherman M, Mazzaferro V, Bolondi L, Craxi A, et al. Efficacy and safety of sorafenib in patients with advanced hepatocellular carcinoma: subanalyses of a phase III trial. J Hepatol. 2012 Oct;57((4)):821–9. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

