Abnormal pattern of brain glucose metabolism in Parkinson's disease: replication in three European cohorts
- PMID: 31768600
- PMCID: PMC6974499
- DOI: 10.1007/s00259-019-04570-7
Abnormal pattern of brain glucose metabolism in Parkinson's disease: replication in three European cohorts
Abstract
Rationale: In Parkinson's disease (PD), spatial covariance analysis of 18F-FDG PET data has consistently revealed a characteristic PD-related brain pattern (PDRP). By quantifying PDRP expression on a scan-by-scan basis, this technique allows objective assessment of disease activity in individual subjects. We provide a further validation of the PDRP by applying spatial covariance analysis to PD cohorts from the Netherlands (NL), Italy (IT), and Spain (SP).
Methods: The PDRPNL was previously identified (17 controls, 19 PD) and its expression was determined in 19 healthy controls and 20 PD patients from the Netherlands. The PDRPIT was identified in 20 controls and 20 "de-novo" PD patients from an Italian cohort. A further 24 controls and 18 "de-novo" Italian patients were used for validation. The PDRPSP was identified in 19 controls and 19 PD patients from a Spanish cohort with late-stage PD. Thirty Spanish PD patients were used for validation. Patterns of the three centers were visually compared and then cross-validated. Furthermore, PDRP expression was determined in 8 patients with multiple system atrophy.
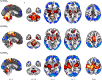
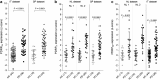
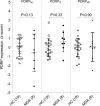
Results: A PDRP could be identified in each cohort. Each PDRP was characterized by relative hypermetabolism in the thalamus, putamen/pallidum, pons, cerebellum, and motor cortex. These changes co-varied with variable degrees of hypometabolism in posterior parietal, occipital, and frontal cortices. Frontal hypometabolism was less pronounced in "de-novo" PD subjects (Italian cohort). Occipital hypometabolism was more pronounced in late-stage PD subjects (Spanish cohort). PDRPIT, PDRPNL, and PDRPSP were significantly expressed in PD patients compared with controls in validation cohorts from the same center (P < 0.0001), and maintained significance on cross-validation (P < 0.005). PDRP expression was absent in MSA.
Conclusion: The PDRP is a reproducible disease characteristic across PD populations and scanning platforms globally. Further study is needed to identify the topography of specific PD subtypes, and to identify and correct for center-specific effects.
Keywords: 18F-FDG PET; Metabolic pattern; Networks; Parkinson’s disease.
Conflict of interest statement
The authors declare that they have no conflicts of interest.
Figures



References
-
- Palop JJ, Chin J, Mucke L. A network dysfunction perspective on neurodegenerative diseases. Nature. 2006;443:768–773. - PubMed
-
- Reivich M, Kuhl D, Wolf A, Greenberg J, Phelps M, Ido T, Casella V, Fowler J, Hoffman E, Alavi A, Som P, Sokoloff L. The [18F]fluorodeoxyglucose method for the measurement of local cerebral glucose utilization in man. Circulation Research. 1979;44(1):127–137. - PubMed
-
- Juh R, Kim J, Moon D, Choe B, Suh T. Different metabolic patterns analysis of Parkinsonism on the 18F-FDG PET. Eur J Radiol. 2004;51:223–233. - PubMed
-
- Eckert Thomas, Barnes Anna, Dhawan Vijay, Frucht Steve, Gordon Mark F., Feigin Andrew S., Eidelberg D. FDG PET in the differential diagnosis of parkinsonian disorders. NeuroImage. 2005;26(3):912–921. - PubMed
-
- Teune Laura K., Bartels Anna L., de Jong Bauke M., Willemsen Antoon T. M., Eshuis Silvia A., de Vries Jeroen J., van Oostrom Joost C. H., Leenders Klaus L. Typical cerebral metabolic patterns in neurodegenerative brain diseases. Movement Disorders. 2010;25(14):2395–2404. - PubMed

