Transition in the etiology of liver cirrhosis in Japan: a nationwide survey
- PMID: 31768801
- PMCID: PMC7026312
- DOI: 10.1007/s00535-019-01645-y
Transition in the etiology of liver cirrhosis in Japan: a nationwide survey
Abstract
Background: To assess the recent real-world changes in the etiologies of liver cirrhosis (LC) in Japan, we conducted a nationwide survey in the annual meeting of the Japan Society of Hepatology (JSH).
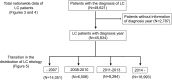
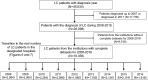
Methods: We investigated the etiologies of LC patients accumulated from 68 participants in 79 institutions (N = 48,621). We next assessed changing trends in the etiologies of LC by analyzing cases in which the year of diagnosis was available (N = 45,834). We further evaluated the transition in the real number of newly identified LC patients by assessing data from 36 hospitals with complete datasets for 2008-2016 (N = 18,358).
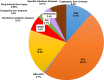
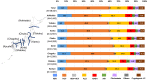
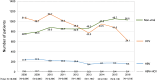
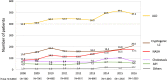
Results: In the overall data, HCV infection (48.2%) was the leading cause of LC in Japan, and HBV infection (11.5%) was the third-most common cause. Regarding the transition in the etiologies of LC, the contribution of viral hepatitis-related LC dropped from 73.4 to 49.7%. Among the non-viral etiologies, alcoholic-related disease (ALD) and nonalcoholic steatohepatitis (NASH)-related LC showed a notable increase (from 13.7 to 24.9% and from 2.0 to 9.1%, respectively). Regarding the real numbers of newly diagnosed patients from 2008 to 2016, the numbers of patients with viral hepatitis-related LC decreased, while the numbers of patients with non-viral LC increased.
Conclusions: HCV has remained the main cause of LC in Japan; however, the contribution of viral hepatitis as an etiology of LC is suggested to have been decreasing. In addition, non-viral LC, such as ALD-related LC and NASH-related LC, is suggested to have increased as etiologies of LC in Japan.
Keywords: Cirrhosis; Etiology; Nationwide survey; Viral hepatitis.
Conflict of interest statement
Yoshiyuki Ueno received honoraria from AbbVie and EA-Pharma. Koichi Takaguchi has received honoraria from AbbVie. Masaki Kurosaki received honoraria from Gilead, AbbVie, Bayer, Otsuka and Eisai. Tatsuya Kanto received honoraria from MSD and Gilead. Shuhei Nishiguchi received honoraria from AbbVie and Gilead and research grants from AbbVie, Toray and EA-Pharma. The other authors have no conflicts of interest to disclose.
Figures

References
-
- World Health Organization. https://apps.who.int/iris/bitstream/handle/10665/246177/WHO-HIV-2016.06-.... Accessed 5 Aug 2019.
MeSH terms
LinkOut - more resources
Full Text Sources
Medical

