The Effect of 4-Methylpyrazole on Oxidative Metabolism of Acetaminophen in Human Volunteers
- PMID: 31768936
- PMCID: PMC7099124
- DOI: 10.1007/s13181-019-00740-z
The Effect of 4-Methylpyrazole on Oxidative Metabolism of Acetaminophen in Human Volunteers
Abstract
Introduction: Acetaminophen (APAP) is commonly ingested in both accidental and suicidal overdose. Oxidative metabolism by cytochrome P450 2E1 (CYP2E1) produces the hepatotoxic metabolite, N-acetyl-p-benzoquinone imine. CYP2E1 inhibition using 4-methylpyrazole (4-MP) has been shown to prevent APAP-induced liver injury in mice and human hepatocytes. This study was conducted to assess the effect of 4-MP on APAP metabolism in humans.
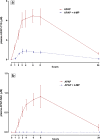
Methods: This crossover trial examined the ability of 4-MP to inhibit CYP2E1 metabolism of APAP in five human volunteers. Participants received a single oral dose of APAP 80 mg/kg, both with and without intravenous 4-MP, after which urinary and plasma oxidative APAP metabolites were measured. The primary outcome was the fraction of ingested APAP excreted as total oxidative metabolites (APAP-CYS, APAP-NAC, APAP-GSH).
Results: Compared with APAP alone, co-treatment with 4-MP decreased the percentage of ingested APAP recovered as oxidative metabolites in 24-hour urine from 4.48 to 0.51% (95% CI = 2.31-5.63%, p = 0.003). Plasma concentrations of these oxidative metabolites also decreased.
Conclusions: These results show 4-MP effectively reduced oxidative metabolism of APAP in human volunteers ingesting a supratherapeutic APAP dose.
Trial registration: ClinicalTrials.gov Identifier: NCT03878693.
Keywords: 4-Methylpyrazole; Acetaminophen toxicity; CYP2E1; Hepatotoxicity; Overdose.
Conflict of interest statement
None.
Figures



References
-
- Salmonson H, Sjoberg G, Brogren J. The standard treatment protocol for paracetamol poisoning may be inadequate following overdose with modified release formulation: a pharmacokinetic and clinical analysis of 53 cases. Clin Toxicol (Phila) 2018;56(1):63–68. - PubMed
-
- Cairney DG, Beckwith HK, Al-Hourani K, Eddleston M, Bateman DN, Dear JW. Plasma paracetamol concentration at hospital presentation has a dose-dependent relationship with liver injury despite prompt treatment with intravenous acetylcysteine. Clin Toxicol (Phila) 2016;54(5):405–410. - PubMed
-
- Abernethy DR, Greenblatt DJ, Divoll M, Ameer B, Shader RI. Differential effect of cimetidine on drug oxidation (antipyrine and diazepam) vs. conjugation (acetaminophen and lorazepam): prevention of acetaminophen toxicity by cimetidine. J Pharmacol Exp Ther. 1983;224(3):508–513. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

