Novel IDH1-Targeted Glioma Therapies
- PMID: 31768950
- PMCID: PMC7027940
- DOI: 10.1007/s40263-019-00684-6
Novel IDH1-Targeted Glioma Therapies
Abstract
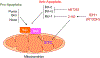
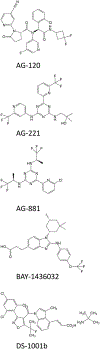
Mutations in the isocitrate dehydrogenase (IDH) 1 gene are commonly found in human glioma, with the majority of low-grade gliomas harboring a recurrent point mutation (IDH1 R132H). Mutant IDH reveals an altered enzymatic activity leading to the synthesis of 2-hydroxyglutarate, which has been implicated in epigenetic mechanisms of oncogenesis. Nevertheless, it is unclear exactly how IDH mutations drive glioma initiation and progression, and it is also not clear why tumors with this mutation generally have a better prognosis than IDH wild-type tumors. Recognition of the high frequency of IDH mutations in glioma [and also in other malignancies, including acute myeloid leukemia (AML) and cholangiocarcinoma] have led to the development of a number of targeted agents that can inhibit these enzymes. Enasidenib and ivosidenib have both gained regulatory approval for IDH mutant AML. Both agents are still in early clinical phases for glioma therapy, as are a number of additional candidates (including AG-881, BAY1436032, and DS1001). A marked clinical problem in the development of these agents is overcoming the blood-brain barrier. An alternative approach to target the IDH1 mutation is by the induction of synthetic lethality with compounds that target poly (ADP-ribose) polymerase (PARP), glutamine metabolism, and the Bcl-2 family of proteins. We conclude that within the last decade, several approaches have been devised to therapeutically target the IDH1 mutation, and that, potentially, both IDH1 inhibitors and synthetic lethal approaches might be relevant for future therapies.
Conflict of interest statement
Conflicts of interest
Georg Karpel-Massler, Trang Thi Thu Nguyen, Enyuan Shang and Markus D. Siegelin declare that they have no conflict of interest that might be relevant to the contents of this manuscript.
Figures

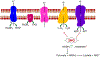
References
-
- Sjoblom T, Jones S, Wood LD, Parsons DW, Lin J, Barber TD, et al. The consensus coding sequences of human breast and colorectal cancers. Science. 2006. October 13;314(5797):268–74. - PubMed
-
- Miller JJ, Shih HA, Andronesi OC, Cahill DP. Isocitrate dehydrogenase-mutant glioma: Evolving clinical and therapeutic implications. Cancer. 2017. December 1;123(23):4535–46. - PubMed
-
- Hartmann C, Meyer J, Balss J, Capper D, Mueller W, Christians A, et al. Type and frequency of IDH1 and IDH2 mutations are related to astrocytic and oligodendroglial differentiation and age: a study of 1,010 diffuse gliomas. Acta Neuropathol. 2009. October;118(4):469–74. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

