Genomic foundations of evolution and ocular pathogenesis in human adenovirus species D
- PMID: 31769017
- PMCID: PMC7185199
- DOI: 10.1002/1873-3468.13693
Genomic foundations of evolution and ocular pathogenesis in human adenovirus species D
Abstract
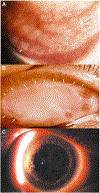
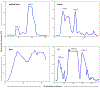
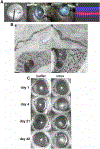
Human adenovirus commonly causes infections of respiratory, gastrointestinal, genitourinary, and ocular surface mucosae. Although most adenovirus eye infections are mild and self-limited, specific viruses within human adenovirus species D are associated with epidemic keratoconjunctivitis (EKC), a severe and highly contagious ocular surface infection, which can lead to chronic and/or recurrent, visually disabling keratitis. In this review, we discuss the links between adenovirus ontogeny, genomics, immune responses, and corneal pathogenesis, for those viruses that cause EKC.
Keywords: conjunctivitis; epidemic keratoconjunctivitis; genomics; homologous recombination; human adenovirus; inflammation; ocular infection; pink eye; tropism; virus evolution.
© 2019 Federation of European Biochemical Societies.
Figures



References
-
- Harrach B, Benkö M, Both GW, Brown M, Davison AJ, Echavarría M, Hess M, Jones MS, Kajon A, Lehmkuhl HD, Mautner V, Mittal SK and Wadell G (2012) Family - Adenoviridae In Virus taxonomy : classification and nomenclature of viruses : ninth report of the International Committee on Taxonomy of Viruses (Andrew MQ King MJA, Carstens Eric B., and Lefkowitz Elliot J., ed.êds), pp. 125–141. Elsevier, Oxford United Kingdom, Amsterdam.
-
- Trentin JJ, Yabe Y and Taylor G (1962). The quest for human cancer viruses. Science 137, 835–41. - PubMed
-
- Chow LT, Gelinas RE, Broker TR and Roberts RJ (1977). An amazing sequence arrangement at the 5’ ends of adenovirus 2 messenger RNA. Cell 12, 1–8. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

