High Circulation of Malaria and Low Prevalence of Bacteremia in Febrile and Afebrile Children in Northeastern Gabon
- PMID: 31769404
- PMCID: PMC6947801
- DOI: 10.4269/ajtmh.19-0368
High Circulation of Malaria and Low Prevalence of Bacteremia in Febrile and Afebrile Children in Northeastern Gabon
Abstract
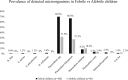
The epidemiology of febrile illness etiologies is under-explored in resource-poor settings. Establishing a local repertory of microorganisms circulating in blood of febrile and afebrile people is important for physicians. Blood was collected from 428 febrile and 88 afebrile children in Makokou (Gabon) and analyzed using polymerase chain reaction. Plasmodium spp. were the pathogens, which were most detected in febrile children (69.6%; 298/428) and in afebrile children (31.8%; 28/88) (P < 0.0001). Plasmodium falciparum was the most prevalent species in both febrile and afebrile children (66.8% and 27.3%, respectively). No differences were observed between febrile and afebrile children for Plasmodium malariae and Plasmodium ovale (8.2% versus 10.2% and 3.3% versus 3.4%, respectively). Triple infection with P. falciparum, P. malariae, and P. ovale was also detected in 1% of febrile children (4/428). Filariasis due to Mansonella perstans was detected in 10 febrile patients (2.3%), whereas Loa loa was detected in both febrile and afebrile children (1.4% and 2.3%, respectively). Bacterial DNA was detected in only 4.4% (19/428) of febrile children, including 13 (68.4%) who were coinfected with at least one Plasmodium species. These were Haemophilus influenzae (1.6%, 7/428), Streptococcus pneumoniae and Staphylococcus aureus (1.2%, 5/428), and Rickettsia felis (0.9%, 4/428). Coxiella burnetii, Bartonella spp., Borrelia spp., Tropheryma whipplei, Anaplasma spp., Leptospira spp., Streptococcus pyogenes, and Salmonella spp. were not detected. This study also highlights the over-prescription and the overuse of antibiotics and antimalarials. Overall, malaria remains a major health problem in Makokou. Malaria control measures must be reconsidered in this region.
Conflict of interest statement
Disclosure: Written informed consent from the legal guardian or parents was obtained for each child included. Information collected from participants was treated confidentially, and the data were anonymized. Funding sources played no role in the design and conduct of the study (collection, management, analysis, and interpretation of the data and preparation, review, or approval of the manuscript). All data generated and material used during this study are included in this published article and its supplementary information. This study was approved by the Gabon National Committee for Research Ethics (CNER) (No. 0020/2015/SG/CNE).
Figures




Similar articles
-
Co-circulation of Plasmodium and Bacterial DNAs in Blood of Febrile and Afebrile Children from Urban and Rural Areas in Gabon.Am J Trop Med Hyg. 2016 Jul 6;95(1):123-32. doi: 10.4269/ajtmh.15-0751. Epub 2016 Apr 25. Am J Trop Med Hyg. 2016. PMID: 27114297 Free PMC article.
-
Molecular Detection of Fastidious and Common Bacteria as Well as Plasmodium spp. in Febrile and Afebrile Children in Franceville, Gabon.Am J Trop Med Hyg. 2015 May;92(5):926-32. doi: 10.4269/ajtmh.14-0699. Epub 2015 Mar 23. Am J Trop Med Hyg. 2015. PMID: 25802432 Free PMC article.
-
Assessment of the burden of malaria and bacteraemia by retrospective molecular diagnosis in febrile illnesses and first-line anti-infectives in Côte d'Ivoire.Travel Med Infect Dis. 2021 Sep-Oct;43:102105. doi: 10.1016/j.tmaid.2021.102105. Epub 2021 Jun 17. Travel Med Infect Dis. 2021. PMID: 34146685
-
Severe febrile illness in adult hospital admissions in Tanzania: a prospective study in an area of high malaria transmission.Trans R Soc Trop Med Hyg. 2012 Nov;106(11):688-95. doi: 10.1016/j.trstmh.2012.08.006. Epub 2012 Sep 28. Trans R Soc Trop Med Hyg. 2012. PMID: 23022040 Review.
-
Management of the febrile child without a focus of infection in the era of universal pneumococcal immunization.Pediatr Infect Dis J. 2002 Jun;21(6):584-8; discussion 613-4. doi: 10.1097/00006454-200206000-00033. Pediatr Infect Dis J. 2002. PMID: 12182394 Review.
Cited by
-
Asymptomatic Malaria Infection and Hidden Parasitic Burden in Gabonese Schoolchildren: Unveiling Silent Co-Infections in Rural and Urban Settings.Trop Med Infect Dis. 2024 Dec 31;10(1):11. doi: 10.3390/tropicalmed10010011. Trop Med Infect Dis. 2024. PMID: 39852662 Free PMC article.
-
The Prevalence of Malaria and Bacteremia Co-Infections among Febrile Patients: A Systematic Review and Meta-Analysis.Trop Med Infect Dis. 2022 Sep 13;7(9):243. doi: 10.3390/tropicalmed7090243. Trop Med Infect Dis. 2022. PMID: 36136654 Free PMC article. Review.
-
Prevalence, probability, and characteristics of malaria and filariasis co-infections: A systematic review and meta-analysis.PLoS Negl Trop Dis. 2022 Oct 21;16(10):e0010857. doi: 10.1371/journal.pntd.0010857. eCollection 2022 Oct. PLoS Negl Trop Dis. 2022. PMID: 36269701 Free PMC article.
-
Decrease on malaria clinical cases from 2017 to 2019 in Franceville, Southeast Gabon, Central Africa.J Public Health Afr. 2023 May 4;14(3):1865. doi: 10.4081/jphia.2023.1865. eCollection 2023 Mar 31. J Public Health Afr. 2023. PMID: 37229438 Free PMC article.
-
Real-time PCR for malaria diagnosis and identification of Plasmodium species in febrile patients in Cubal, Angola.Parasit Vectors. 2024 Sep 11;17(1):384. doi: 10.1186/s13071-024-06467-3. Parasit Vectors. 2024. PMID: 39261971 Free PMC article.
References
-
- World Health Organization , 2018. Maternal, Newborn, Child and Adolescent Health. Geneva, Switzerland: WHO.
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

