In vivo safety profile of a CSPG4-directed IgE antibody in an immunocompetent rat model
- PMID: 31769737
- PMCID: PMC6927758
- DOI: 10.1080/19420862.2019.1685349
In vivo safety profile of a CSPG4-directed IgE antibody in an immunocompetent rat model
Abstract
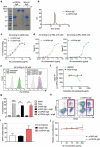
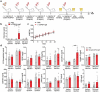
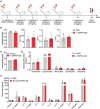
IgE monoclonal antibodies hold great potential for cancer therapy. Preclinical in vivo systems, particularly those in which the antibody recognizes the host species target antigen and binds to cognate Fc receptors, are often the closest approximation to human exposure and represent a key challenge for evaluating the safety of antibody-based therapies. We sought to develop an immunocompetent rat system to assess the safety of a rodent anti-tumor IgE, as a surrogate for the human therapeutic candidate. We generated a rat IgE against the human tumor-associated antigen chondroitin sulfate proteoglycan 4 (CSPG4) and cross-reactive for the rat antigen. We analyzed CSPG4 distribution in normal rat and human tissues and investigated the in vivo safety of the antibody by monitoring clinical signs and molecular biomarkers after systemic administration to immunocompetent rats. Human and rat CSPG4 expression in normal tissues were comparable. Animals receiving antibody exhibited transient mild to moderate adverse events accompanied by mild elevation of serum tryptase, but not of angiotensin II or cytokines implicated in allergic reactions or cytokine storm. In the long term, repeated antibody administration was well tolerated, with no changes in animal body weight, liver and kidney functions or blood cell counts. This model provides preclinical support for the safety profiling of IgE therapeutic antibodies. Due to the comparable antigen tissue distribution in human and rat, this model may also comprise an appropriate tool for proof-of-concept safety evaluations of different treatment approaches targeting CSPG4.
Keywords: CSPG4; IgE; allergooncology; antibody; immunotherapy; rat model; species cross-reactivity.
Figures


References
-
- Burgess M, Mapp S, Mazzieri R, Cheung C, Chambers L, Mattarollo SR, Mollee P, Gill D, Saunders NA. Increased FcgammaRIIB dominance contributes to the emergence of resistance to therapeutic antibodies in chronic lymphocytic leukaemia patients. Oncogene. 2017;36:2366–76. doi: 10.1038/onc.2016.387. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
