Electron Paramagnetic Resonance Spectroscopic Identification of the Fe-S Clusters in the SPASM Domain-Containing Radical SAM Enzyme PqqE
- PMID: 31769977
- PMCID: PMC6959549
- DOI: 10.1021/acs.biochem.9b00960
Electron Paramagnetic Resonance Spectroscopic Identification of the Fe-S Clusters in the SPASM Domain-Containing Radical SAM Enzyme PqqE
Abstract
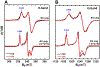
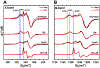
Pyrroloquinoline quinone (PQQ) is an important redox active quinocofactor produced by a wide variety of bacteria. A key step in PQQ biosynthesis is a carbon-carbon cross-link reaction between glutamate and tyrosine side chains within the ribosomally synthesized peptide substrate PqqA. This reaction is catalyzed by the radical SAM enzyme PqqE. Previous X-ray crystallographic and spectroscopic studies suggested that PqqE, like the other members of the SPASM domain family, contains two auxiliary Fe-S clusters (AuxI and AuxII) in addition to the radical SAM [4Fe-4S] cluster. However, a clear assignment of the electron paramagnetic resonance (EPR) signal of each Fe-S cluster was hindered by the isolation of a His6-tagged PqqE variant with an altered AuxI cluster. In this work, we are able to isolate soluble PqqE variants by using a less disruptive strep-tactin chromatographic approach. We have unambiguously identified the EPR signatures for four forms of Fe-S clusters present in PqqE through the use of multifrequency EPR spectroscopy: the RS [4Fe-4S] cluster, the AuxII [4Fe-4S] cluster, and two different clusters ([4Fe-4S] and [2Fe-2S]) bound in the AuxI site. The RS [4Fe-4S] cluster, the AuxII [4Fe-4S] cluster, and the [2Fe-2S] cluster form in the AuxI site can all be reduced by sodium dithionite, with g tensors of their reduced form determined as [2.040, 1.927, 1.897], [2.059, 1.940, 1.903], and [2.004, 1.958, 1.904], respectively. The AuxI [4Fe-4S] cluster that is determined on the basis of its relaxation profile can be reduced only by using low-potential reductants such as Ti(III) citrate or Eu(II)-DTPA to give rise to a g1 = 2.104 signal. Identification of the EPR signature for each cluster paves the way for further investigations of SPASM domain radical SAM enzymes.
Figures










References
-
- Duine JA (1999) The PQQ story, J. Biosci. Bioeng 88, 231–236. - PubMed
-
- Anthony C (2001) Pyrroloquinoline quinone (PQQ) and quinoprotein enzymes, Antioxid. Redox Signal 3, 757–774. - PubMed
-
- Goodwin PM, and Anthony C (1998) The biochemistry, physiology and genetics of PQQ and PQQ-containing enzymes, In Advances in Microbial Physiology (Poole RK, Ed.), pp 1–80, Academic Press. - PubMed
-
- Sode K, Ito K, Witarto AB, Watanabe K, Yoshida H, and Postma P (1996) Increased production of recombinant pyrroloquinoline quinone (PQQ) glucose dehydrogenase by metabolically engineered Escherichia coli strain capable of PQQ biosynthesis, J. Biotechnol 49, 239–243. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

