Systematically Prioritizing Candidates in Genome-Based Drug Repurposing
- PMID: 31769998
- PMCID: PMC6921094
- DOI: 10.1089/adt.2019.950
Systematically Prioritizing Candidates in Genome-Based Drug Repurposing
Abstract
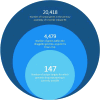
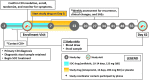
Drug repurposing is the application of approved drugs to treat diseases separate and distinct from their original indications. Herein, we define the scope of all practical precision drug repurposing using DrugBank, a publicly available database of pharmacological agents, and BioVU, a large, de-identified DNA repository linked to longitudinal electronic health records at Vanderbilt University Medical Center. We present a method of repurposing candidate prioritization through integration of pharmacodynamic and marketing variables from DrugBank with quality control thresholds for genomic data derived from the DNA samples within BioVU. Through the synergy of delineated "target-action pairs," along with target genomics, we identify ∼230 "pairs" that represent all practical opportunities for genomic drug repurposing. From this analysis, we present a pipeline of 14 repurposing candidates across 7 disease areas that link to our repurposability platform and present high potential for randomized controlled trial startup in upcoming months.
Keywords: drug repurposing; genomics; informatics; phenome-wide association study (PheWAS); precision medicine.
Conflict of interest statement
The authors declare no competing interests.
Figures

References
-
- Nosengo N: Can you teach old drugs new tricks? Nature 2016;534:314–316 - PubMed
-
- Scannell JW, Blanckley A, Boldon H, Warrington B: Diagnosing the decline in pharmaceutical R&D efficiency. Nat Rev Drug Discov 2012;11:191–200 - PubMed
-
- Teva Pharmaceuticals and IBM Expand Global Partnership to Enable Drug Development and Chronic Disease Management with Watson. www.tevapharm.com/news/teva_pharmaceuticals_and_ibm_expand_global_partne... (Last accessed on April13, 2018)
-
- Bayer, Broad Expand Partnership to Advance Cancer Drug Discovery Research. Broad Institute (2017). https://www.broadinstitute.org/news/bayer-broad-expand-partnership-advan... (Last accessed on April17, 2018)
Appendix References
-
- Homo sapiens—Ensembl genome browser 94. <ext-link xmlns:xlink="http://www.w3.org/1999/xlink" ext-link-type="uri" xlink:href="http://useast.ensembl.org/Homo_AU12sapiens/Info/Annotation">https://useast.ensembl.org/Homo_ AU12sapiens/Info/Annotation</ext-link> (Last accessed on January2, 2019)
-
- PROCLAIM—Misoprostol in the prevention of recurrent CDI prevent recurrence of Clostridium difficile infection with misoprostol—full text view—Clinical Trials.gov. https://clinicaltrials.gov/ct2/show/NCT03617172 (Last accessed on November9, 2018)
-
- Documentation and Sources—DrugBank. https://www.drugbank.ca/documentation (Last accessed on April27, 2018)
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

