Clade F AAVHSCs cross the blood brain barrier and transduce the central nervous system in addition to peripheral tissues following intravenous administration in nonhuman primates
- PMID: 31770409
- PMCID: PMC6879147
- DOI: 10.1371/journal.pone.0225582
Clade F AAVHSCs cross the blood brain barrier and transduce the central nervous system in addition to peripheral tissues following intravenous administration in nonhuman primates
Abstract
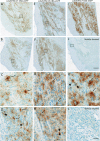
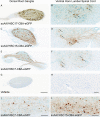
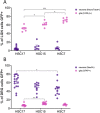
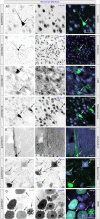
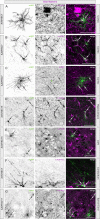
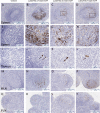
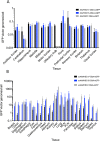
The biodistribution of AAVHSC7, AAVHSC15, and AAVHSC17 following systemic delivery was assessed in cynomolgus macaques (Macaca fascicularis). Animals received a single intravenous (IV) injection of a self-complementary AAVHSC-enhanced green fluorescent protein (eGFP) vector and tissues were harvested at two weeks post-dose for anti-eGFP immunohistochemistry and vector genome analyses. IV delivery of AAVHSC vectors produced widespread distribution of eGFP staining in glial cells throughout the central nervous system, with the highest levels seen in the pons and lateral geniculate nuclei (LGN). eGFP-positive neurons were also observed throughout the central and peripheral nervous systems for all three AAVHSC vectors including brain, spinal cord, and dorsal root ganglia (DRG) with staining evident in neuronal cell bodies, axons and dendritic arborizations. Co-labeling of sections from brain, spinal cord, and DRG with anti-eGFP antibodies and cell-specific markers confirmed eGFP-staining in neurons and glia, including protoplasmic and fibrous astrocytes and oligodendrocytes. For all capsids tested, 50 to 70% of glial cells (S100-β+) and on average 8% of neurons (NeuroTrace+) in the LGN were positive for eGFP expression. In the DRG, 45 to 62% of neurons and 8 to 12% of satellite cells were eGFP-positive for the capsids tested. eGFP staining was also observed in peripheral tissues with abundant staining in hepatocytes, skeletal- and cardio-myocytes and in acinar cells of the pancreas. Biodistribution of AAVHSC vector genomes in the central and peripheral organs generally correlated with eGFP staining and were highest in the liver for all AAVHSC vectors tested. These data demonstrate that AAVHSCs have broad tissue tropism and cross the blood-nerve and blood-brain-barriers following systemic delivery in nonhuman primates, making them suitable gene editing or gene transfer vectors for therapeutic application in human genetic diseases.
Conflict of interest statement
The authors have read the journal's policy and the authors [JLE, JG, LJS, HR, TAS, KP, NZ, KO, MO, AS] of this manuscript have the following competing interests: All authors are current or former employees of Homology Medicines, Inc. EC is an employee of Premier Laboratory, LLC and performed immunohistochemistry under contract with Homology Medicines, Inc. PM is an employee of The Mannheimer Foundation Inc. and performed in-life biodistribution studies in nonhuman primates under contract with Homology Medicines, Inc. The commercial affiliation between Homology Medicines, Inc. and Premier Laboratory LLC and between Homology Medicines, Inc., and the Mannheimer Foundation, Inc. does not alter our adherence to all PLOS ONE policies on sharing data and materials.
Figures












References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

