LFRET, a novel rapid assay for anti-tissue transglutaminase antibody detection
- PMID: 31770411
- PMCID: PMC6879146
- DOI: 10.1371/journal.pone.0225851
LFRET, a novel rapid assay for anti-tissue transglutaminase antibody detection
Abstract
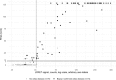
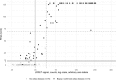
The diagnosis of celiac disease (CD) is currently based on serology and intestinal biopsy, with detection of anti-tissue transglutaminase (tTG) IgA antibodies recommended as the first-line test. Emphasizing the increasing importance of serological testing, new guidelines and evidence suggest basing the diagnosis solely on serology without confirmatory biopsy. Enzyme immunoassays (EIAs) are the established approach for anti-tTG antibody detection, with the existing point-of-care (POC) tests lacking sensitivity and/or specificity. Improved POC methods could help reduce the underdiagnosis and diagnostic delay of CD. We have previously developed rapid homogenous immunoassays based on time-resolved Förster resonance energy transfer (TR-FRET), and demonstrated their suitability in serodiagnostics with hanta- and Zika virus infections as models. In this study, we set out to establish a protein L -based TR-FRET assay (LFRET) for the detection of anti-tTG antibodies. We studied 74 patients with biopsy-confirmed CD and 70 healthy controls, with 1) the new tTG-LFRET assay, and for reference 2) a well-established EIA and 3) an existing commercial POC test. IgG depletion was employed to differentiate between anti-tTG IgA and IgG positivity. The sensitivity and specificity of the first-generation tTG-LFRET POC assay in detection of CD were 87.8% and 94.3%, respectively, in line with those of the reference POC test. The sensitivity and specificity of EIA were 95.9% and 91.9%, respectively. This study demonstrates the applicability of LFRET to serological diagnosis of autoimmune diseases in general and of CD in particular.
Conflict of interest statement
Some of the authors are inventors in a patent "Protein L based bioassay method for determining presence of soluble antibodies in a sample and kit therefore (WO2015128548)" owned by University of Helsinki describing the LFRET assay utilized in the manuscript. (https://patentscope.wipo.int/search/en/detail.jsf?docId=WO2015128548). This does not alter our adherence to PLOS ONE policies on sharing data and materials and all authors declare that the work was carried out in accordance with good research ethics.
Figures


References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

