Synthesis of (-)-Mitrephorone A via a Bioinspired Late Stage C-H Oxidation of (-)-Mitrephorone B
- PMID: 31770485
- PMCID: PMC6946608
- DOI: 10.1021/jacs.9b11646
Synthesis of (-)-Mitrephorone A via a Bioinspired Late Stage C-H Oxidation of (-)-Mitrephorone B
Abstract
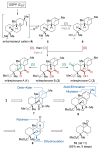
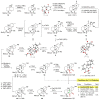
We present a bioinspired late-stage C-H oxidation of the ent-trachylobane natural product mitrephorone B to mitrephorone A. The realization of this unprecedented transformation was accomplished by either an iron-catalyzed or electrochemical oxidation and enabled access to the densely substituted oxetane in one step. Formation of mitrephorone C, which is lacking the central oxetane unit but features a keto-function at C2, was not formed under these conditions.
Conflict of interest statement
The authors declare no competing financial interest.
Figures
References
-
- Hanson JR. Diterpenoids. Nat Prod Rep. 2006;23:875–885. - PubMed
- Hanson JR. Diterpenoids. Nat Prod Rep. 2007;24:1332–1341. - PubMed
- Roy A, Roberts FG, Wilderman PR, Zhou K, Peters RJ, Coates RM. 16-Aza-ent-beyerane and 16-Aza-ent-trachylobane: potent mechanism-based inhibitors of recombinant ent-kaurene synthase from Arabidopsis thaliana. J Am Chem Soc. 2007;129:12453–12460. - PMC - PubMed
-
- Hong YJ, Tantillo DJ. Formation of beyerene, kaurene, trachylobane, and atiserene diterpenes by rearrangements that avoid secondary carbocations. J Am Chem Soc. 2010;132:5375–5386. - PubMed
-
- Hugelshofer CL, Magauer T. A Bioinspired Cyclization Sequence Enables the Asymmetric Total Synthesis of Dictyoxetane. J Am Chem Soc. 2016;138:6420–6423. - PubMed
- Hugelshofer CL, Magauer T. A Divergent Approach to the Marine Diterpenoids (+)-Dictyoxetane and (+)-Dolabellane V. Chem Eur J. 2016;22:15125–15136. - PubMed
-
- Richter MJR, Schneider M, Brandstätter M, Krautwald S, Carreira EM. Total Synthesis of (–)-Mitrephorone A. J Am Chem Soc. 2018;140:16704–16710. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

