Glucose Transport and Transporters in the Endomembranes
- PMID: 31771288
- PMCID: PMC6929180
- DOI: 10.3390/ijms20235898
Glucose Transport and Transporters in the Endomembranes
Abstract
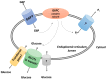
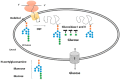
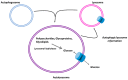
Glucose is a basic nutrient in most of the creatures; its transport through biological membranes is an absolute requirement of life. This role is fulfilled by glucose transporters, mediating the transport of glucose by facilitated diffusion or by secondary active transport. GLUT (glucose transporter) or SLC2A (Solute carrier 2A) families represent the main glucose transporters in mammalian cells, originally described as plasma membrane transporters. Glucose transport through intracellular membranes has not been elucidated yet; however, glucose is formed in the lumen of various organelles. The glucose-6-phosphatase system catalyzing the last common step of gluconeogenesis and glycogenolysis generates glucose within the lumen of the endoplasmic reticulum. Posttranslational processing of the oligosaccharide moiety of glycoproteins also results in intraluminal glucose formation in the endoplasmic reticulum (ER) and Golgi. Autophagic degradation of polysaccharides, glycoproteins, and glycolipids leads to glucose accumulation in lysosomes. Despite the obvious necessity, the mechanism of glucose transport and the molecular nature of mediating proteins in the endomembranes have been hardly elucidated for the last few years. However, recent studies revealed the intracellular localization and functional features of some glucose transporters; the aim of the present paper was to summarize the collected knowledge.
Keywords: GLUT; SGLT; endomembrane; glucose; transporter.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

