Effect of calcium on the interaction of Acinetobacter baumannii with human respiratory epithelial cells
- PMID: 31771504
- PMCID: PMC6880639
- DOI: 10.1186/s12866-019-1643-z
Effect of calcium on the interaction of Acinetobacter baumannii with human respiratory epithelial cells
Abstract
Background: Investigating the factors that influence Acinetobacter baumannii(Ab) adhesion/invasion of host cells is important to understand its pathogenicity. Metal cations have been shown to play an important role in regulating the biofilm formation and increasing the virulence of Ab; however, the effect of calcium on host-bacterial interaction has yet to be clarified. Here, the dynamic process of the interaction between Ab and human respiratory epithelial cells and the effect of calcium on host-bacterial interaction were explored using microscopic imaging, quantitative PCR and real time cellular analysis (RTCA).
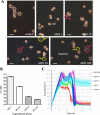
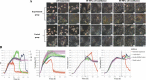
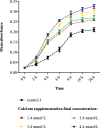
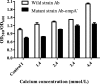
Results: The concentration of calcium, multiplicity of infection and co-culture time were all demonstrated to have effects on host-bacterial interaction. A unique "double peak" phenomenon changed to a sharp "single peak" phenomenon during the process of Ab infection under the effect of calcium was observed in the time-dependent cell response profiles. Moreover, calcium can increase Ab adhesion/invasion of epithelial cells by regulating the expression of Ab-related genes (ompA, bfmRS, abaI).
Conclusions: Effective control of calcium concentrations can provide new approaches for the prevention and treatment of multi-drug resistant Ab.
Keywords: Acinetobacter baumannii; Calcium; host-bacterial interaction; Infection.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures


Similar articles
-
Regulation of Acinetobacter baumannii biofilm formation.Future Microbiol. 2009 Apr;4(3):273-8. doi: 10.2217/fmb.09.5. Future Microbiol. 2009. PMID: 19327114 Free PMC article.
-
Contribution of the A. baumannii A1S_0114 Gene to the Interaction with Eukaryotic Cells and Virulence.Front Cell Infect Microbiol. 2017 Apr 3;7:108. doi: 10.3389/fcimb.2017.00108. eCollection 2017. Front Cell Infect Microbiol. 2017. PMID: 28421168 Free PMC article.
-
Capacity of multidrug-resistant clinical isolates of Acinetobacter baumannii to form biofilm and adhere to epithelial cell surfaces.Clin Microbiol Infect. 2008 Jan;14(1):49-54. doi: 10.1111/j.1469-0691.2007.01842.x. Epub 2007 Nov 13. Clin Microbiol Infect. 2008. PMID: 18005176
-
[Current approaches to explain the virulence of Acinetobacter baumannii].Mikrobiyol Bul. 2011 Apr;45(2):371-80. Mikrobiyol Bul. 2011. PMID: 21644082 Review. Turkish.
-
Biofilm formation in Acinetobacter baumannii.New Microbiol. 2014 Apr;37(2):119-27. Epub 2014 Apr 1. New Microbiol. 2014. PMID: 24858639 Review.
Cited by
-
Effect of Hcp Iron Ion Regulation on the Interaction Between Acinetobacter baumannii With Human Pulmonary Alveolar Epithelial Cells and Biofilm Formation.Front Cell Infect Microbiol. 2022 Feb 23;12:761604. doi: 10.3389/fcimb.2022.761604. eCollection 2022. Front Cell Infect Microbiol. 2022. PMID: 35281445 Free PMC article.
-
A novel method for evaluating the dynamic biocompatibility of degradable biomaterials based on real-time cell analysis.Regen Biomater. 2020 Jun;7(3):321-329. doi: 10.1093/rb/rbaa017. Epub 2020 May 1. Regen Biomater. 2020. PMID: 32523733 Free PMC article.
-
Transcriptomic Analysis of Mycobacterial Infected Macrophages Reveals a High MOI Specific Type I IFN Signaling.Infect Immun. 2023 Jul 18;91(7):e0015523. doi: 10.1128/iai.00155-23. Epub 2023 Jun 20. Infect Immun. 2023. PMID: 37338365 Free PMC article.
-
Alleviating penicillin-resistant Streptococcus pneumoniae‑induced lung epithelial cell injury: mechanistic insights into effects of the optimized combination of main components from Yinhuapinggan granules.BMC Infect Dis. 2025 Apr 20;25(1):565. doi: 10.1186/s12879-025-10951-1. BMC Infect Dis. 2025. PMID: 40254610 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

