Clinical pilot study to evaluate the neovaginal PACIENA prosthesis® for vaginoplasty without skin grafts in women with vaginal agenesis
- PMID: 31771581
- PMCID: PMC6880434
- DOI: 10.1186/s12905-019-0841-z
Clinical pilot study to evaluate the neovaginal PACIENA prosthesis® for vaginoplasty without skin grafts in women with vaginal agenesis
Abstract
Background: To evaluate the feasibility and clinical outcomes of vaginoplasties using a neovaginal polylactic acid prosthesis made with 3-dimensional (3D) printing technology as an intraneovaginal mould.
Methods: This was an interventionist, prospective, and multicentre clinical pilot investigation of a sanitary product (PACIENA prosthesis®) aiming to recruit and operate on 8 patients over 6 months with a follow-up period of 6 months. Only six patients with Rokitansky syndrome and one patient with Morris syndrome (7 patients in total) were operated on in two university hospitals: "La Fe", Valencia (H1) and "Arrixaca", Murcia (H2).
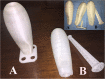
Interventions: Extensive surgical dissection of a defined space between the urethra and bladder in the front and of the rectum in the back as well as insertion of the PACIENA prosthesis® covered with Interceed® were performed. After 12 days, the prosthesis was changed to the silicone-covered version for daily application.
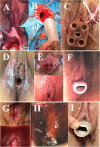
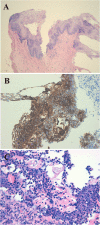
Results: In the 6 patients with Rokitansky syndrome (86%), the primary endpoint (satisfactory vaginal outcome in terms of appearance, function, and sensation without relevant additional morbidity) was achieved, although only 2 patients (28%) were sexually active at the end of 6 months of follow-up. The patient with Morris syndrome withdrew from the study after 1 month. Patients without bacterial colonization showed positive Schiller tests at 1 month, and subsequent biopsies showed adequate keratinization and epidermization. Epithelization and iodopositivity were delayed in the patients who developed inflammatory granulomas.
Conclusions: Good anatomical and functional results can be achieved with the PACIENA prosthesis® for vaginoplasties without skin grafts. However, adequate patient selection and education, good surgical techniques and haemostasis, postoperative support, and prevention of bacterial colonization are important.
Trial registration: This clinical study was approved by the Ethical Clinical Investigation Committee of San Juan University Hospital on September 27, 2016, to be conducted in the participating centres; it was authorized by the Spanish Agency of Medicines and Health Products (AEMPS) on April 24, 2017 (exp. no. 585/16/EC), to be carried out in that hospitals.
Keywords: Neovaginal prosthesis; Rokitansky syndrome; Vaginal agenesis; Vaginoplasty.
Conflict of interest statement
The authors declare that they have no competing financial interests.
Figures
Similar articles
-
Classical McIndoe Technique Versus the McIndoe Technique with a Neovaginal PACIENA Prosthesis® and No Skin Graft.J Clin Med. 2020 Nov 13;9(11):3648. doi: 10.3390/jcm9113648. J Clin Med. 2020. PMID: 33202784 Free PMC article.
-
Vaginoplasty with oxidized cellulose: anatomical, functional and histological evaluation.Eur J Obstet Gynecol Reprod Biol. 2012 Aug;163(2):204-9. doi: 10.1016/j.ejogrb.2012.04.018. Epub 2012 Jun 26. Eur J Obstet Gynecol Reprod Biol. 2012. PMID: 22739655
-
Novel Minimally Invasive Technique of Neovaginoplasty Using an Absorbable Adhesion Barrier.J Minim Invasive Gynecol. 2020 Jan;27(1):206-211. doi: 10.1016/j.jmig.2019.02.025. Epub 2019 Jun 19. J Minim Invasive Gynecol. 2020. PMID: 31228594
-
Vaginal replacement in the pediatric age group: a 34-year experience of intestinal vaginoplasty in children and young girls.J Pediatr Surg. 2010 Oct;45(10):2087-91. doi: 10.1016/j.jpedsurg.2010.05.016. J Pediatr Surg. 2010. PMID: 20920736 Review.
-
Vaginoplasty with an Autologous Buccal Mucosa Fenestrated Graft in Two Patients with Vaginal Agenesis: A Multidisciplinary Approach and Literature Review.J Minim Invasive Gynecol. 2017 May-Jun;24(4):670-676. doi: 10.1016/j.jmig.2016.12.030. Epub 2017 Feb 15. J Minim Invasive Gynecol. 2017. PMID: 28212868 Free PMC article. Review.
Cited by
-
Classical McIndoe Technique Versus the McIndoe Technique with a Neovaginal PACIENA Prosthesis® and No Skin Graft.J Clin Med. 2020 Nov 13;9(11):3648. doi: 10.3390/jcm9113648. J Clin Med. 2020. PMID: 33202784 Free PMC article.
-
Development and Applications of Organoids in Gynecological Diseases.Stem Cell Rev Rep. 2025 Apr;21(3):629-644. doi: 10.1007/s12015-024-10833-0. Epub 2024 Dec 12. Stem Cell Rev Rep. 2025. PMID: 39666266 Free PMC article. Review.
-
Extracellular Matrix-Based and Electrospun Scaffolding Systems for Vaginal Reconstruction.Bioengineering (Basel). 2023 Jul 1;10(7):790. doi: 10.3390/bioengineering10070790. Bioengineering (Basel). 2023. PMID: 37508817 Free PMC article. Review.
-
Genitourinary Tissue Engineering: Reconstruction and Research Models.Bioengineering (Basel). 2021 Jul 13;8(7):99. doi: 10.3390/bioengineering8070099. Bioengineering (Basel). 2021. PMID: 34356206 Free PMC article. Review.
-
Histologic Analysis of 'Distraction Vaginogenesis' in a Rat Model.Pathophysiology. 2024 Jun 8;31(2):298-308. doi: 10.3390/pathophysiology31020022. Pathophysiology. 2024. PMID: 38921727 Free PMC article.
References
-
- McIndoe A. The treatment of congenital absence and obliterative conditions of the vagina. Br J Plast Surg. 1950;2:254–267. - PubMed
-
- Garcia J, Jones HW. The split thickness graft technic for vaginal agenesis. Obstet Gynecol. 1977;49:328–332. - PubMed
-
- Brucker SY, Gegusch M, Zubke W, Rall K, Gauwerky JF, Wallwiener D. Neovagina creation in vaginal agenesis: development of a new laparoscopic Vecchietti-based procedure and optimized instruments in a prospective comparative interventional study in 101 patients. Fertil Steril. 2008;90:1940–1952. doi: 10.1016/j.fertnstert.2007.08.070. - DOI - PubMed
Publication types
MeSH terms
Substances
Supplementary concepts
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

