Paradoxical clade- and age-specific vaccine effectiveness during the 2018/19 influenza A(H3N2) epidemic in Canada: potential imprint-regulated effect of vaccine (I-REV)
- PMID: 31771709
- PMCID: PMC6864978
- DOI: 10.2807/1560-7917.ES.2019.24.46.1900585
Paradoxical clade- and age-specific vaccine effectiveness during the 2018/19 influenza A(H3N2) epidemic in Canada: potential imprint-regulated effect of vaccine (I-REV)
Abstract
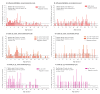
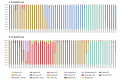
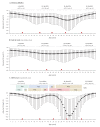
IntroductionThe Canadian Sentinel Practitioner Surveillance Network reports vaccine effectiveness (VE) for the 2018/19 influenza A(H3N2) epidemic.AimTo explain a paradoxical signal of increased clade 3C.3a risk among 35-54-year-old vaccinees, we hypothesise childhood immunological imprinting and a cohort effect following the 1968 influenza A(H3N2) pandemic.MethodsWe assessed VE by test-negative design for influenza A(H3N2) overall and for co-circulating clades 3C.2a1b and 3C.3a. VE variation by age in 2018/19 was compared with amino acid variation in the haemagglutinin glycoprotein by year since 1968.ResultsInfluenza A(H3N2) VE was 17% (95% CI: -13 to 39) overall: 27% (95% CI: -7 to 50) for 3C.2a1b and -32% (95% CI: -119 to 21) for 3C.3a. Among 20-64-year-olds, VE was -7% (95% CI: -56 to 26): 6% (95% CI: -49 to 41) for 3C.2a1b and -96% (95% CI: -277 to -2) for 3C.3a. Clade 3C.3a VE showed a pronounced negative dip among 35-54-year-olds in whom the odds of medically attended illness were > 4-fold increased for vaccinated vs unvaccinated participants (p < 0.005). This age group was primed in childhood to influenza A(H3N2) viruses that for two decades following the 1968 pandemic bore a serine at haemagglutinin position 159, in common with contemporary 3C.3a viruses but mismatched to 3C.2a vaccine strains instead bearing tyrosine.DiscussionImprinting by the first childhood influenza infection is known to confer long-lasting immunity focused toward priming epitopes. Our findings suggest vaccine mismatch may negatively interact with imprinted immunity. The immunological mechanisms for imprint-regulated effect of vaccine (I-REV) warrant investigation.
Keywords: A(H3N2); Influenza; clade; cohort effect; imprinting; vaccine effectiveness.
Conflict of interest statement
Figures

References
-
- Skowronski DM, Leir S, De Serres G, Murti M, Dickinson JA, Winter A-L, et al. Children under 10 years of age were more affected by the 2018/19 influenza A(H1N1)pdm09 epidemic in Canada: possible cohort effect following the 2009 influenza pandemic. Euro Surveill. 2019;24(15):1900104. 10.2807/1560-7917.ES.2019.24.15.1900104 - DOI - PMC - PubMed
-
- Public Health Agency of Canada (PHAC). Influenza weekly reports 2018-19 season. FluWatch report: April 28 to May 4, 2019 (Week 18). Ottawa: PHAC; 2019. Available from: https://www.canada.ca/en/public-health/services/diseases/flu-influenza/i...
-
- World Health Organization (WHO). WHO recommendations on the composition of influenza virus vaccines Geneva: WHO. [Accessed: 29 Oct 2019]. Available from: https://www.who.int/influenza/vaccines/virus/recommendations/en/
-
- Worldwide Influenza Centre, Francis Crick Institute. Annual and interim reports. London: Francis Crick Institute. [Accessed: 29 Oct 2019]. Available from: https://www.crick.ac.uk/research/worldwide-influenza-centre/annual-and-i...
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
