Shoulder Injury Related to Vaccine Administration (SIRVA): Petitioner claims to the National Vaccine Injury Compensation Program, 2010-2016
- PMID: 31771864
- PMCID: PMC9169064
- DOI: 10.1016/j.vaccine.2019.11.032
Shoulder Injury Related to Vaccine Administration (SIRVA): Petitioner claims to the National Vaccine Injury Compensation Program, 2010-2016
Abstract
Background: Since 2010, petitioner claims of shoulder injury related to vaccine administration (SIRVA) to the National Vaccine Injury Compensation Program (VICP) have been increasing.
Objective: To conduct a scientific review of clinical characteristics of SIRVA petitions to the VICP.
Methods: We queried the VICP's Injury Compensation System database for medical reports of alleged SIRVA and SIRVA-like injuries. Medical reports are summaries of petitioner claims and supporting documentation along with a VICP clinician reviewer diagnosis and assessment of criteria for concession. We conducted a descriptive analysis of SIRVA petitioner claims recommended by the VICP for concession as SIRVA injuries.
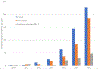
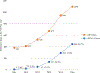
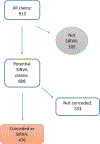
Results: We identified 476 petitioner claims recommended for concession. Claims per year increased from two in 2011, the first full year in the analytic period, to 227 in 2016. Median age was 51 years, 82.8% were women, and median body mass index was 25.1 (range 17.0-48.9). Four hundred cases (84.0%) involved influenza vaccine. Pharmacy or store (n = 168; 35.3%) was the most common place of vaccination followed by doctor's office (n = 147; 30.9%). Fewer than half of cases reported a suspected administration error; 172 (36.1%) reported 'injection too high' on the arm. Shoulder pain, rotator cuff problems, and bursitis were common initial diagnoses. Most (80.0%) cases received physical or occupational therapy, 60.1% had at least one steroid injection, and 32.6% had surgery. Most (71.9%) healthcare providers who gave opinions on causality considered the injury was caused by vaccination. A minority (24.3%) of cases indicated that symptoms had resolved by the last visit available in medical records.
Conclusions: Most conceded claims for SIRVA were in women and involved influenza vaccines. Injection too high on the arm could be a factor due to the risk of injecting into underlying non-muscular tissues. Healthcare providers should be aware of proper injection technique and anatomical landmarks when administering vaccines.
Keywords: Concession; National Vaccine Injury Compensation Program (VICP); Petitioner claims; Shoulder injury; Shoulder injury related to vaccine administration (SIRVA); Vaccination.
Copyright © 2019 Elsevier Ltd. All rights reserved.
Conflict of interest statement
Declaration of Competing Interest The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures
References
-
- Cook KM, Evans G. The national vaccine injury compensation program. Pediatrics 2011;127(Suppl 1):S74–7. - PubMed
-
- Atanasoff S, Ryan T, Lightfoot R, Johann-Liang R. Shoulder injury related to vaccine administration (SIRVA). Vaccine 2010;28:8049–52. - PubMed
-
- Bodor M, Montalvo E. Vaccination-related shoulder dysfunction. Vaccine 2007;25:585–7. - PubMed
-
- Uchida S, Sakai A, Nakamura T. Subacromial bursitis following human papilloma virus vaccine misinjection. Vaccine 2012;31:27–30. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

