Solenodon genome reveals convergent evolution of venom in eulipotyphlan mammals
- PMID: 31772017
- PMCID: PMC6926037
- DOI: 10.1073/pnas.1906117116
Solenodon genome reveals convergent evolution of venom in eulipotyphlan mammals
Abstract
Venom systems are key adaptations that have evolved throughout the tree of life and typically facilitate predation or defense. Despite venoms being model systems for studying a variety of evolutionary and physiological processes, many taxonomic groups remain understudied, including venomous mammals. Within the order Eulipotyphla, multiple shrew species and solenodons have oral venom systems. Despite morphological variation of their delivery systems, it remains unclear whether venom represents the ancestral state in this group or is the result of multiple independent origins. We investigated the origin and evolution of venom in eulipotyphlans by characterizing the venom system of the endangered Hispaniolan solenodon (Solenodon paradoxus). We constructed a genome to underpin proteomic identifications of solenodon venom toxins, before undertaking evolutionary analyses of those constituents, and functional assessments of the secreted venom. Our findings show that solenodon venom consists of multiple paralogous kallikrein 1 (KLK1) serine proteases, which cause hypotensive effects in vivo, and seem likely to have evolved to facilitate vertebrate prey capture. Comparative analyses provide convincing evidence that the oral venom systems of solenodons and shrews have evolved convergently, with the 4 independent origins of venom in eulipotyphlans outnumbering all other venom origins in mammals. We find that KLK1s have been independently coopted into the venom of shrews and solenodons following their divergence during the late Cretaceous, suggesting that evolutionary constraints may be acting on these genes. Consequently, our findings represent a striking example of convergent molecular evolution and demonstrate that distinct structural backgrounds can yield equivalent functions.
Keywords: convergent molecular evolution; gene duplication; genotype phenotype; kallikrein toxin; venom systems.
Copyright © 2019 the Author(s). Published by PNAS.
Conflict of interest statement
The authors declare no competing interest.
Figures

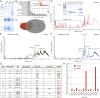

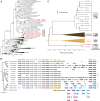
References
-
- Casewell N. R., Wüster W., Vonk F. J., Harrison R. A., Fry B. G., Complex cocktails: The evolutionary novelty of venoms. Trends Ecol. Evol. 28, 219–229 (2013). - PubMed
-
- Fry B. G., et al. , The toxicogenomic multiverse: Convergent recruitment of proteins into animal venoms. Annu. Rev. Genomics Hum. Genet. 10, 483–511 (2009). - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources

