Shaping the zebrafish myotome by intertissue friction and active stress
- PMID: 31772022
- PMCID: PMC6925982
- DOI: 10.1073/pnas.1900819116
Shaping the zebrafish myotome by intertissue friction and active stress
Abstract
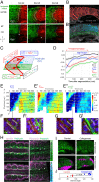
Organ formation is an inherently biophysical process, requiring large-scale tissue deformations. Yet, understanding how complex organ shape emerges during development remains a major challenge. During zebrafish embryogenesis, large muscle segments, called myotomes, acquire a characteristic chevron morphology, which is believed to aid swimming. Myotome shape can be altered by perturbing muscle cell differentiation or the interaction between myotomes and surrounding tissues during morphogenesis. To disentangle the mechanisms contributing to shape formation of the myotome, we combine single-cell resolution live imaging with quantitative image analysis and theoretical modeling. We find that, soon after segmentation from the presomitic mesoderm, the future myotome spreads across the underlying tissues. The mechanical coupling between the future myotome and the surrounding tissues appears to spatially vary, effectively resulting in spatially heterogeneous friction. Using a vertex model combined with experimental validation, we show that the interplay of tissue spreading and friction is sufficient to drive the initial phase of chevron shape formation. However, local anisotropic stresses, generated during muscle cell differentiation, are necessary to reach the acute angle of the chevron in wild-type embryos. Finally, tissue plasticity is required for formation and maintenance of the chevron shape, which is mediated by orientated cellular rearrangements. Our work sheds light on how a spatiotemporal sequence of local cellular events can have a nonlocal and irreversible mechanical impact at the tissue scale, leading to robust organ shaping.
Keywords: organ morphogenesis; somitogenesis; tissue mechanics; vertex models.
Copyright © 2019 the Author(s). Published by PNAS.
Conflict of interest statement
The authors declare no competing interest.
Figures



Similar articles
-
Chevron formation of the zebrafish muscle segments.J Exp Biol. 2014 Nov 1;217(Pt 21):3870-82. doi: 10.1242/jeb.102202. Epub 2014 Sep 29. J Exp Biol. 2014. PMID: 25267843 Free PMC article.
-
Vertebrate myotome development.Birth Defects Res C Embryo Today. 2005 Sep;75(3):172-9. doi: 10.1002/bdrc.20046. Birth Defects Res C Embryo Today. 2005. PMID: 16187310 Review.
-
Signals that instruct somite and myotome formation persist in Xenopus laevis early tailbud stage embryos.Cells Tissues Organs. 2002;172(1):1-12. doi: 10.1159/000064387. Cells Tissues Organs. 2002. PMID: 12364823
-
Somitogenesis.Curr Top Dev Biol. 1998;38:225-87. Curr Top Dev Biol. 1998. PMID: 9399080 Review.
-
Friction forces position the neural anlage.Nat Cell Biol. 2017 Apr;19(4):306-317. doi: 10.1038/ncb3492. Epub 2017 Mar 27. Nat Cell Biol. 2017. PMID: 28346437 Free PMC article.
Cited by
-
Spinal cord elongation enables proportional regulation of the zebrafish posterior body.Development. 2025 Jan 1;152(1):dev204438. doi: 10.1242/dev.204438. Epub 2025 Jan 9. Development. 2025. PMID: 39745249 Free PMC article.
-
Differentiation and Maturation of Muscle and Fat Cells in Cultivated Seafood: Lessons from Developmental Biology.Mar Biotechnol (NY). 2023 Feb;25(1):1-29. doi: 10.1007/s10126-022-10174-4. Epub 2022 Nov 14. Mar Biotechnol (NY). 2023. PMID: 36374393 Free PMC article. Review.
-
wnt16 regulates spine and muscle morphogenesis through parallel signals from notochord and dermomyotome.PLoS Genet. 2022 Nov 8;18(11):e1010496. doi: 10.1371/journal.pgen.1010496. eCollection 2022 Nov. PLoS Genet. 2022. PMID: 36346812 Free PMC article.
-
The zebrafish presomitic mesoderm elongates through compaction-extension.Cells Dev. 2021 Dec;168:203748. doi: 10.1016/j.cdev.2021.203748. Epub 2021 Sep 28. Cells Dev. 2021. PMID: 34597846 Free PMC article.
-
Mechanical stress driven by rigidity sensing governs epithelial stability.Nat Phys. 2023 Jan;19:132-141. doi: 10.1038/s41567-022-01826-2. Epub 2022 Nov 28. Nat Phys. 2023. PMID: 36686215 Free PMC article.
References
-
- Baena-López L. A., Baonza A., García-Bellido A., The orientation of cell divisions determines the shape of Drosophila organs. Curr. Biol. 15, 1640–1644 (2005). - PubMed
-
- Hozumi S., et al. , An unconventional myosin in Drosophila reverses the default handedness in visceral organs. Nature 440, 798–802 (2006). - PubMed
-
- Affolter M., Zeller R., Caussinus E., Tissue remodelling through branching morphogenesis. Nat. Rev. Mol. Cell Biol. 10, 831–842 (2009). - PubMed
-
- Hamant O., et al. , Developmental patterning by mechanical signals in Arabidopsis. Science 322, 1650–1655 (2008). - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

