FKBPL-based peptide, ALM201, targets angiogenesis and cancer stem cells in ovarian cancer
- PMID: 31772325
- PMCID: PMC7000737
- DOI: 10.1038/s41416-019-0649-5
FKBPL-based peptide, ALM201, targets angiogenesis and cancer stem cells in ovarian cancer
Abstract
Background: ALM201 is a therapeutic peptide derived from FKBPL that has previously undergone preclinical and clinical development for oncology indications and has completed a Phase 1a clinical trial in ovarian cancer patients and other advanced solid tumours.
Methods: In vitro, cancer stem cell (CSC) assays in a range of HGSOC cell lines and patient samples, and in vivo tumour initiation, growth delay and limiting dilution assays, were utilised. Mechanisms were determined by using immunohistochemistry, ELISA, qRT-PCR, RNAseq and western blotting. Endogenous FKBPL protein levels were evaluated using tissue microarrays (TMA).
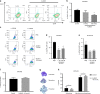
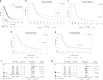
Results: ALM201 reduced CSCs in cell lines and primary samples by inducing differentiation. ALM201 treatment of highly vascularised Kuramochi xenografts resulted in tumour growth delay by disruption of angiogenesis and a ten-fold decrease in the CSC population. In contrast, ALM201 failed to elicit a strong antitumour response in non-vascularised OVCAR3 xenografts, due to high levels of IL-6 and vasculogenic mimicry. High endogenous tumour expression of FKBPL was associated with an increased progression-free interval, supporting the protective role of FKBPL in HGSOC.
Conclusion: FKBPL-based therapy can (i) dually target angiogenesis and CSCs, (ii) target the CD44/STAT3 pathway in tumours and (iii) is effective in highly vascularised HGSOC tumours with low levels of IL-6.
Conflict of interest statement
T.R. is an inventor and patent holder for ALM201. T.R. has received research funding from Alamc Discovery Ltd. A.de.F. has received a grant from AstraZeneca (unrelated to work in this paper). The remaining authors declare no competing interests.
Figures




References
-
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2015. CA Cancer J. Clin. 2015;65:5–29. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous

