Atherosclerosis imaging with 18F-sodium fluoride PET: state-of-the-art review
- PMID: 31773235
- PMCID: PMC7188711
- DOI: 10.1007/s00259-019-04603-1
Atherosclerosis imaging with 18F-sodium fluoride PET: state-of-the-art review
Abstract
Purpose: We examined the literature to elucidate the role of 18F-sodium fluoride (NaF)-PET in atherosclerosis.
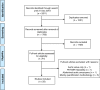
Methods: Following a systematic search of PubMed/MEDLINE, Embase, and Cochrane Library included articles underwent subjective quality assessment with categories low, medium, and high. Of 2811 records, 1780 remained after removal of duplicates. Screening by title and abstract left 41 potentially eligible full-text articles, of which 8 (about the aortic valve (n = 1), PET/MRI feasibility (n = 1), aortic aneurysms (n = 1), or quantification methodology (n = 5)) were dismissed, leaving 33 published 2010-2012 (n = 6), 2013-2015 (n = 11), and 2016-2018 (n = 16) for analysis.
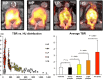
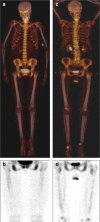
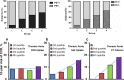
Results: They focused on coronary (n = 8), carotid (n = 7), and femoral arteries (n = 1), thoracic aorta (n = 1), and infrarenal aorta (n = 1). The remaining 15 studies examined more than one arterial segment. The literature was heterogeneous: few studies were designed to investigate atherosclerosis, 13 were retrospective, 9 applied both FDG and NaF as tracers, 24 NaF only. Subjective quality was low in one, medium in 13, and high in 19 studies. The literature indicates that NaF is a very specific tracer that mimics active arterial wall microcalcification, which is positively associated with cardiovascular risk. Arterial NaF uptake often presents before CT-calcification, tends to decrease with increasing density of CT-calcification, and appears, rather than FDG-avid foci, to progress to CT-calcification. It is mainly surface localized, increases with age with a wide scatter but without an obvious sex difference. NaF-avid microcalcification can occur in fatty streaks, but the degree of progression to CT-calcification is unknown. It remains unknown whether medical therapy influences microcalcification. The literature held no therapeutic or randomized controlled trials.
Conclusion: The literature was heterogeneous and with few clear cut messages. NaF-PET is a new approach to detect and quantify microcalcification in early-stage atherosclerosis. NaF uptake correlates with cardiovascular risk factors and appears to be a good measure of the body's atherosclerotic burden, potentially suited also for assessment of anti-atherosclerotic therapy.
Keywords: 18F-sodium fluoride; Atherosclerosis; Calcification; NaF; PET; Quantification.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures
References
-
- Worlds Health Organization. Cardiovascular disease. WHO 2019. https://www.who.int/cardiovascular_diseases/en/. Accessed 15 Sep 2019.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

