Chemoenzymatic Semisynthesis of Proteins
- PMID: 31774265
- PMCID: PMC7101271
- DOI: 10.1021/acs.chemrev.9b00450
Chemoenzymatic Semisynthesis of Proteins
Abstract
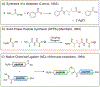
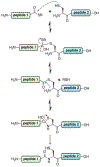
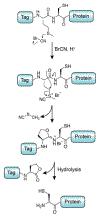
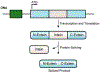
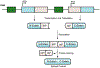
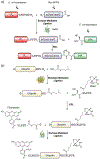
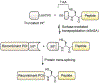
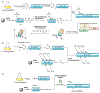
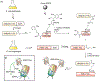
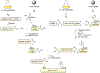
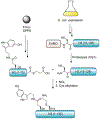
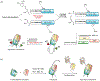
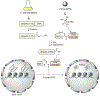
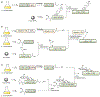
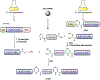
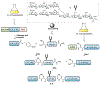
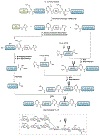
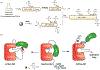
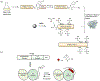
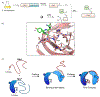
Protein semisynthesis-defined herein as the assembly of a protein from a combination of synthetic and recombinant fragments-is a burgeoning field of chemical biology that has impacted many areas in the life sciences. In this review, we provide a comprehensive survey of this area. We begin by discussing the various chemical and enzymatic methods now available for the manufacture of custom proteins containing noncoded elements. This section begins with a discussion of methods that are more chemical in origin and ends with those that employ biocatalysts. We also illustrate the commonalities that exist between these seemingly disparate methods and show how this is allowing for the development of integrated chemoenzymatic methods. This methodology discussion provides the technical foundation for the second part of the review where we cover the great many biological problems that have now been addressed using these tools. Finally, we end the piece with a short discussion on the frontiers of the field and the opportunities available for the future.
Figures
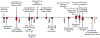
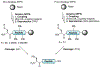



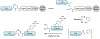

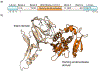

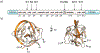




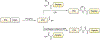
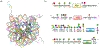
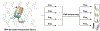




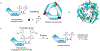


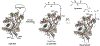

References
-
- International Human Genome Sequencing, C., Finishing the Euchromatic Sequence of the Human Genome. Nature 2004, 431, 931–945. - PubMed
-
- Boutureira O; Bernardes GJL, Advances in Chemical Protein Modification. Chem. Rev 2015, 115, 2174–2195. - PubMed
-
- Lang K; Chin JW, Cellular Incorporation of Unnatural Amino Acids and Bioorthogonal Labeling of Proteins. Chem. Rev 2014, 114, 4764–4806. - PubMed
-
- Kent SBH, Total Chemical Synthesis of Proteins. Chem. Soc. Rev 2009, 38, 338–351. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

