Two π-Electrons Make the Difference: From BODIPY to BODIIM Switchable Fluorescent Dyes
- PMID: 31774591
- PMCID: PMC7027818
- DOI: 10.1002/chem.201905344
Two π-Electrons Make the Difference: From BODIPY to BODIIM Switchable Fluorescent Dyes
Abstract
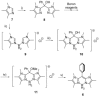
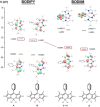
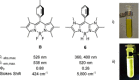
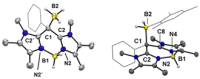
(aza-)BODIPY dyes (boron dipyrromethene dyes) are well-established fluorophores due to their large quantum yields, stability, and diversity, which led to promising applications including imaging techniques, sensors, organic (opto)electronic materials, or biomedical applications. Although the control of the optical properties in (aza-)BODIPY dyes by peripheral functional groups is well studied, we herein present a novel approach to modify the 12 π-electron core of the dipyrromethene scaffold. The replacement of two carbon atoms in the β-position of a BODIPY dye by two nitrogen atoms afforded a 14 π-electron system, which was termed BODIIM (boron diimidazolylmethene) in systematic analogy to the BODIPY dyes. Remarkably, the BODIIM dye was obtained with a BH2 -rigidifying entity, which is currently elusive and highly sought after for the BODIPY dye class. DFT-Calculations confirm the [12+2] π-electron relationship between BODIPY and BODIIM and reveal a strong shape correlation between LUMO in the BODIPY and the HOMO of the BODIIM. The modification of the π-system leads to a dramatic shift of the optical properties, of which the fluorescent emission is most noteworthy and occurs at much larger Stokes shift, that is, ≈500 cm-1 in BODIPY versus >4170 cm-1 in BODIIM system in all solvents investigated. Nucleophilic reactivity was found at the meso-carbon atom in the formation of stable borane adducts with a significant shift of the fluorescent emission, and this behavior contrasts the reactivity of conventional BODIPY systems. In addition, the reverse decomplexation of the borane adducts was demonstrated in reactions with a representative N-heterocyclic carbene to retain the strongly fluorescent BODIIM compound, which suggests applications as fully reversible fluorescent switch.
Keywords: BODIIM; BODIPY; bisimidazoles; fluorescent dyes; switchable fluorescence.
© 2019 The Authors. Published by Wiley-VCH Verlag GmbH & Co. KGaA.
Conflict of interest statement
The authors declare no conflict of interest.
Figures






References
-
- Wood T. E., Thompson A., Chem. Rev. 2007, 107, 1831–1861. - PubMed
-
- For chemical transformations:
-
- Loudet A., Burgess K., Chem. Rev. 2007, 107, 4891–4932; - PubMed
-
- Ulrich G., Ziessel R., Harriman A., Angew. Chem. Int. Ed. 2008, 47, 1184–1201; - PubMed
- Angew. Chem. 2008, 120, 1202–1219;
-
- Boens N., Verbelen B., Dehaen W., Eur. J. Org. Chem. 2015, 6577–6595;
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

