Pembrolizumab for Treatment-Refractory Metastatic Castration-Resistant Prostate Cancer: Multicohort, Open-Label Phase II KEYNOTE-199 Study
- PMID: 31774688
- PMCID: PMC7186583
- DOI: 10.1200/JCO.19.01638
Pembrolizumab for Treatment-Refractory Metastatic Castration-Resistant Prostate Cancer: Multicohort, Open-Label Phase II KEYNOTE-199 Study
Abstract
Purpose: Pembrolizumab has previously shown antitumor activity against programmed death ligand 1 (PD-L1)-positive metastatic castration-resistant prostate cancer (mCRPC). Here, we assessed the antitumor activity and safety of pembrolizumab in three parallel cohorts of a larger mCRPC population.
Methods: The phase II KEYNOTE-199 study included three cohorts of patients with mCRPC treated with docetaxel and one or more targeted endocrine therapies. Cohorts 1 and 2 enrolled patients with RECIST-measurable PD-L1-positive and PD-L1-negative disease, respectively. Cohort 3 enrolled patients with bone-predominant disease, regardless of PD-L1 expression. All patients received pembrolizumab 200 mg every 3 weeks for up to 35 cycles. The primary end point was objective response rate per RECIST v1.1 assessed by central review in cohorts 1 and 2. Secondary end points included disease control rate, duration of response, overall survival (OS), and safety.
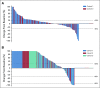
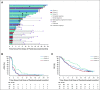
Results: Two hundred fifty-eight patients were enrolled: 133 in cohort 1, 66 in cohort 2, and 59 in cohort 3. Objective response rate was 5% (95% CI, 2% to 11%) in cohort 1 and 3% (95% CI, < 1% to 11%) in cohort 2. Median duration of response was not reached (range, 1.9 to ≥ 21.8 months) and 10.6 months (range, 4.4 to 16.8 months), respectively. Disease control rate was 10% in cohort 1, 9% in cohort 2, and 22% in cohort 3. Median OS was 9.5 months in cohort 1, 7.9 months in cohort 2, and 14.1 months in cohort 3. Treatment-related adverse events occurred in 60% of patients, were of grade 3 to 5 severity in 15%, and led to discontinuation of treatment in 5%.
Conclusion: Pembrolizumab monotherapy shows antitumor activity with an acceptable safety profile in a subset of patients with RECIST-measurable and bone-predominant mCRPC previously treated with docetaxel and targeted endocrine therapy. Observed responses seem to be durable, and OS estimates are encouraging.
Trial registration: ClinicalTrials.gov NCT02787005.
Figures
Comment in
-
Pembrolizumab in Metastatic Castration-Resistant Prostate Cancer: Can an Agnostic Become a Believer?J Clin Oncol. 2020 Feb 10;38(5):381-383. doi: 10.1200/JCO.19.02921. Epub 2019 Dec 26. J Clin Oncol. 2020. PMID: 31877088 No abstract available.
-
Re: Pembrolizumab for Treatment-refractory Metastatic Castration-resistant Prostate Cancer: Multicohort, Open-label Phase II KEYNOTE-199 Study.Eur Urol. 2020 Jun;77(6):759-760. doi: 10.1016/j.eururo.2020.02.026. Epub 2020 Feb 27. Eur Urol. 2020. PMID: 32115263 No abstract available.
-
Biomarkers of response to immune checkpoint inhibitors for metastatic castration resistant prostate cancer: looking for the needle in the haystack.Ann Transl Med. 2020 Jul;8(14):894. doi: 10.21037/atm.2020.03.78. Ann Transl Med. 2020. PMID: 32793738 Free PMC article. No abstract available.
References
-
- Gravis G, Fizazi K, Joly F, et al. Androgen-deprivation therapy alone or with docetaxel in non-castrate metastatic prostate cancer (GETUG-AFU 15): A randomised, open-label, phase 3 trial. Lancet Oncol. 2013;14:149–158. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

