Determinants of combination GM-CSF immunotherapy and oncolytic virotherapy success identified through in silico treatment personalization
- PMID: 31774808
- PMCID: PMC6880985
- DOI: 10.1371/journal.pcbi.1007495
Determinants of combination GM-CSF immunotherapy and oncolytic virotherapy success identified through in silico treatment personalization
Abstract
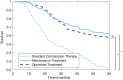
Oncolytic virotherapies, including the modified herpes simplex virus talimogene laherparepvec (T-VEC), have shown great promise as potent instigators of anti-tumour immune effects. The OPTiM trial, in particular, demonstrated the superior anti-cancer effects of T-VEC as compared to systemic immunotherapy treatment using exogenous administration of granulocyte-macrophage colony-stimulating factor (GM-CSF). Theoretically, a combined approach leveraging exogenous cytokine immunotherapy and oncolytic virotherapy would elicit an even greater immune response and improve patient outcomes. However, regimen scheduling of combination immunostimulation and T-VEC therapy has yet to be established. Here, we calibrate a computational biology model of sensitive and resistant tumour cells and immune interactions for implementation into an in silico clinical trial to test and individualize combination immuno- and virotherapy. By personalizing and optimizing combination oncolytic virotherapy and immunostimulatory therapy, we show improved simulated patient outcomes for individuals with late-stage melanoma. More crucially, through evaluation of individualized regimens, we identified determinants of combination GM-CSF and T-VEC therapy that can be translated into clinically-actionable dosing strategies without further personalization. Our results serve as a proof-of-concept for interdisciplinary approaches to determining combination therapy, and suggest promising avenues of investigation towards tailored combination immunotherapy/oncolytic virotherapy.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures


References
-
- Hoster HA, Zanes RP, von Hamm E. The Association of “Viral” Hepatitis and Hodgkin’s Disease. Cancer Res. 1949;9(8):473–480. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

