MS CD49d+CD154+ Lymphocytes Reprogram Oligodendrocytes into Immune Reactive Cells Affecting CNS Regeneration
- PMID: 31775315
- PMCID: PMC6953114
- DOI: 10.3390/cells8121508
MS CD49d+CD154+ Lymphocytes Reprogram Oligodendrocytes into Immune Reactive Cells Affecting CNS Regeneration
Abstract
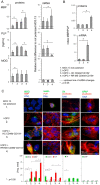
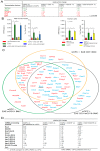
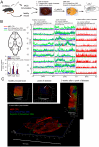
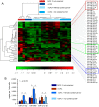
The critical aspect in multiple sclerosis (MS) progression involves insufficient regeneration of CNS resulting from deficient myelin synthesis by newly generated oligodendrocytes (OLs). Although many studies have focused on the role of autoreactive lymphocytes in the inflammatory-induced axonal loss, the problem of insufficient remyelination and disease progression is still unsolved. To determine the effect of myelin-specific lymphocytes on OL function in MS patients and in a mouse model of MS, we cultured myelin induced MS CD49d+CD154+ circulating lymphocytes as well as Experimental Autoimmune Encephalomyelitis (EAE) mouse brain-derived T and memory B cells with maturing oligodendrocyte precursor cells (OPCs). We found that myelin-specific CD49d+CD154+ lymphocytes affected OPC maturation toward formation of immune reactive OLs. Newly generated OLs were characterized by imbalanced myelin basic protein (MBP) and proteolipid protein (PLP) production as well as proinflammatory chemokine/cytokine synthesis. The analysis of cellular pathways responsible for OL reprogramming revealed that CD49d+CD154+ lymphocytes affected miRNA synthesis by dysregulation of polymerase II activity. miR-665 and ELL3 turned out to be the main targets of MS myelin-specific lymphocytes. Neutralization of high intracellular miR-665 concentration restored miRNA and MBP/PLP synthesis. Together, these data point to new targets for therapeutic intervention promoting CNS remyelination.
Keywords: multiple sclerosis; myelin-specific lymphocytes; oligodendrocyte precursor cells; remyelination.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


Similar articles
-
Multiple Sclerosis CD49d+CD154+ As Myelin-Specific Lymphocytes Induced During Remyelination.Cells. 2019 Dec 19;9(1):15. doi: 10.3390/cells9010015. Cells. 2019. PMID: 31861635 Free PMC article.
-
Thymosin beta4 promotes oligodendrogenesis in the demyelinating central nervous system.Neurobiol Dis. 2016 Apr;88:85-95. doi: 10.1016/j.nbd.2016.01.010. Epub 2016 Jan 12. Neurobiol Dis. 2016. PMID: 26805386
-
Early axonal damage and progressive myelin pathology define the kinetics of CNS histopathology in a mouse model of multiple sclerosis.Clin Immunol. 2013 Oct;149(1):32-45. doi: 10.1016/j.clim.2013.06.004. Epub 2013 Jun 18. Clin Immunol. 2013. PMID: 23899992
-
Autoimmune pathogenesis of multiple sclerosis: role of autoreactive T lymphocytes and new immunotherapeutic strategies.Crit Rev Immunol. 1997;17(1):33-75. doi: 10.1615/critrevimmunol.v17.i1.20. Crit Rev Immunol. 1997. PMID: 9034723 Review.
-
Nudging oligodendrocyte intrinsic signaling to remyelinate and repair: Estrogen receptor ligand effects.J Steroid Biochem Mol Biol. 2016 Jun;160:43-52. doi: 10.1016/j.jsbmb.2016.01.006. Epub 2016 Jan 14. J Steroid Biochem Mol Biol. 2016. PMID: 26776441 Free PMC article. Review.
Cited by
-
New insights into the immunologic role of oligodendrocyte lineage cells in demyelination diseases.J Biomed Res. 2022 Apr 28;36(5):343-352. doi: 10.7555/JBR.36.20220016. J Biomed Res. 2022. PMID: 35578762 Free PMC article.
-
Remyelination in multiple sclerosis from the miRNA perspective.Front Mol Neurosci. 2023 Jun 1;16:1199313. doi: 10.3389/fnmol.2023.1199313. eCollection 2023. Front Mol Neurosci. 2023. PMID: 37333618 Free PMC article. Review.
-
Myelin Peptide-Mannan Conjugate Multiple Sclerosis Vaccines: Conjugation Efficacy and Stability of Vaccine Ingredient.Vaccines (Basel). 2021 Dec 8;9(12):1456. doi: 10.3390/vaccines9121456. Vaccines (Basel). 2021. PMID: 34960201 Free PMC article.
-
Multiple Sclerosis CD49d+CD154+ As Myelin-Specific Lymphocytes Induced During Remyelination.Cells. 2019 Dec 19;9(1):15. doi: 10.3390/cells9010015. Cells. 2019. PMID: 31861635 Free PMC article.
-
The Role of MicroRNAs in Repair Processes in Multiple Sclerosis.Cells. 2020 Jul 16;9(7):1711. doi: 10.3390/cells9071711. Cells. 2020. PMID: 32708794 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

