McArdle Disease: New Insights into Its Underlying Molecular Mechanisms
- PMID: 31775340
- PMCID: PMC6929006
- DOI: 10.3390/ijms20235919
McArdle Disease: New Insights into Its Underlying Molecular Mechanisms
Abstract
McArdle disease, also known as glycogen storage disease type V (GSDV), is characterized by exercise intolerance, the second wind phenomenon, and high serum creatine kinase activity. Here, we recapitulate PYGM mutations in the population responsible for this disease. Traditionally, McArdle disease has been considered a metabolic myopathy caused by the lack of expression of the muscle isoform of the glycogen phosphorylase (PYGM). However, recent findings challenge this view, since it has been shown that PYGM is present in other tissues than the skeletal muscle. We review the latest studies about the molecular mechanism involved in glycogen phosphorylase activity regulation. Further, we summarize the expression and functional significance of PYGM in other tissues than skeletal muscle both in health and McArdle disease. Furthermore, we examine the different animal models that have served as the knowledge base for better understanding of McArdle disease. Finally, we give an overview of the latest state-of-the-art clinical trials currently being carried out and present an updated view of the current therapies.
Keywords: McArdle disease; O-glycosylation; glycogen phosphorylase; glycogen storage disease type V; hexosamine biosynthetic pathway; small GTPases.
Conflict of interest statement
The authors declare that they have no conflicts of interest with the contents of this article. All authors qualify for authorship, approved the final version of the manuscript and agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Figures

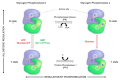
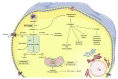

References
-
- McArdle B. Myopathy due to a defect in muscle glycogen breakdown. Clin. Sci. 1951;10:13–35. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- (PRE_2017_1_0016)/A.A.S. is a recipient of a predoctoral fellowship from the Basque Government
- Jesús de Gangoiti y Barrera 2019/M.L.M. is a recipient of a fellowship from Foundation "Jesús de Gangoiti y Barrera".
- (PI18/00207)/J.L.Z. was supported by the Instituto de Salud Carlos III
- (US19/04)/J.L.Z. was supported by University of Basque Country Grant.
LinkOut - more resources
Full Text Sources

