Presynaptic Release-regulating Metabotropic Glutamate Receptors: An Update
- PMID: 31775600
- PMCID: PMC7457419
- DOI: 10.2174/1570159X17666191127112339
Presynaptic Release-regulating Metabotropic Glutamate Receptors: An Update
Abstract
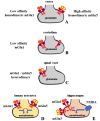
Metabotropic glutamate (mGlu) receptors represent the largest family of glutamate receptors in mammals and act as fine tuners of the chemical transmission in central nervous system (CNS). In the last decade, results concerning the expression and the subcellular localization of mGlu receptors further clarified their role in physio-pathological conditions. Concomitantly, their pharmacological characterization largely improved thanks to the identification of new compounds (chemical ligands and antibodies recognizing epitopic sequences of the receptor proteins) that allowed to decipher the protein compositions of the naive receptors. mGlu receptors are expressed at the presynaptic site of chemical synapses. Here, they modulate intraterminal enzymatic pathways controlling the migration and the fusion of vesicles to synaptic membranes as well as the phosphorylation of colocalized receptors. Both the control of transmitter exocytosis and the phosphorylation of colocalized receptors elicited by mGlu receptors are relevant events that dictate the plasticity of nerve terminals, and account for the main role of presynaptic mGlu receptors as modulators of neuronal signalling. The role of the presynaptic mGlu receptors in the CNS has been the matter of several studies and this review aims at briefly summarizing the recent observations obtained with isolated nerve endings (we refer to as synaptosomes). We focus on the pharmacological characterization of these receptors and on their receptor-receptor interaction / oligo-dimerization in nerve endings that could be relevant to the development of new therapeutic approaches for the cure of central pathologies.
Keywords: Synaptosomes; mGlu1/5; mGlu2/3; mGlu7; oligomerization; presynaptic receptors; receptor-receptor interaction; transmitter release.
Copyright© Bentham Science Publishers; For any queries, please email at epub@benthamscience.net.
Figures

References
-
- Schoepp D.D. Unveiling the functions of presynaptic metabotropic glutamate receptors in the central nervous system. J. Pharmacol. Exp. Ther. 2001;299(1):12–20. - PubMed
-
- Nicoletti F., Battaglia G., Storto M., Ngomba R.T., Iacovelli L., Arcella A., Gradini R., Sale P., Rampello L., De Vita T., Di Marco R., Melchiorri D., Bruno V. Metabotropic glutamate receptors: beyond the regulation of synaptic transmission. Psychoneuroendocrinology. 2007;32(Suppl. 1):S40–S45. doi: 10.1016/j.psyneuen.2007.04.015. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

