Brassinosteroids: Multidimensional Regulators of Plant Growth, Development, and Stress Responses
- PMID: 31776234
- PMCID: PMC7008487
- DOI: 10.1105/tpc.19.00335
Brassinosteroids: Multidimensional Regulators of Plant Growth, Development, and Stress Responses
Abstract
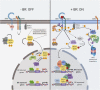
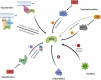
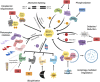
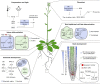
Brassinosteroids (BRs) are a group of polyhydroxylated plant steroid hormones that are crucial for many aspects of a plant's life. BRs were originally characterized for their function in cell elongation, but it is becoming clear that they play major roles in plant growth, development, and responses to several stresses such as extreme temperatures and drought. A BR signaling pathway from cell surface receptors to central transcription factors has been well characterized. Here, we summarize recent progress toward understanding the BR pathway, including BR perception and the molecular mechanisms of BR signaling. Next, we discuss the roles of BRs in development and stress responses. Finally, we show how knowledge of the BR pathway is being applied to manipulate the growth and stress responses of crops. These studies highlight the complex regulation of BR signaling, multiple points of crosstalk between BRs and other hormones or stress responses, and the finely tuned spatiotemporal regulation of BR signaling.
© 2020 American Society of Plant Biologists. All rights reserved.
Figures
References
-
- Amorim-Silva V., García-Moreno Á., Castillo A.G., Lakhssassi N., Esteban Del Valle A., Pérez-Sancho J., Li Y., Posé D., Pérez-Rodriguez J., Lin J., Valpuesta V., Borsani O., et al. (2019). TTL proteins scaffold brassinosteroid signaling components at the plasma membrane to optimize signal transduction in Arabidopsis. Plant Cell 31: 1807–1828. - PMC - PubMed
-
- Anjum S.A., Wang L.C., Farooq M., Hussain M., Xue L.L., Zou C.M. (2011). Brassinolide application improves the drought tolerance in maize through modulation of enzymatic antioxidants and leaf gas exchange. J. Agron. Crop Sci. 197: 177–185.
-
- Anne P., Azzopardi M., Gissot L., Beaubiat S., Hématy K., Palauqui J.C. (2015). OCTOPUS negatively regulates BIN2 to control phloem differentiation in Arabidopsis thaliana. Curr. Biol. 25: 2584–2590. - PubMed
-
- Back T.G., Janzen L., Pharis R.P., Yan Z. (2002). Synthesis and bioactivity of C-2 and C-3 methyl ether derivatives of brassinolide. Phytochemistry 59: 627–634. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

