The 68Ga/177Lu-theragnostic concept in PSMA-targeting of metastatic castration-resistant prostate cancer: impact of post-therapeutic whole-body scintigraphy in the follow-up
- PMID: 31776632
- PMCID: PMC7005064
- DOI: 10.1007/s00259-019-04583-2
The 68Ga/177Lu-theragnostic concept in PSMA-targeting of metastatic castration-resistant prostate cancer: impact of post-therapeutic whole-body scintigraphy in the follow-up
Erratum in
-
Correction to: The 68Ga/177Lu-theragnostic concept in PSMA-targeting of metastatic castration-resistant prostate cancer: impact of post-therapeutic whole-body scintigraphy in the follow-up.Eur J Nucl Med Mol Imaging. 2020 Mar;47(3):740. doi: 10.1007/s00259-019-04660-6. Eur J Nucl Med Mol Imaging. 2020. PMID: 31863137 Free PMC article.
Abstract
Introduction: A new therapeutic option for metastatic castration-resistant prostate cancer (mCRPC) of heavily pre-treated patients lies in 177Lu-PSMA-617 radioligand therapy.
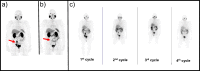
Methods: On the basis of PSMA-targeted 68Ga-PSMA-11 PET/CT, 32 consecutive mCRPC patients were selected for 177Lu-PSMA-617 therapy (6 GBq/cycle, 2 to 6 cycles, 6-10 weeks apart) and followed until death. Post-therapy whole-body (WB) dosimetry and 68Ga-PSMA-11 PET/CT data were compared and related to progression free and overall survival.
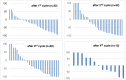
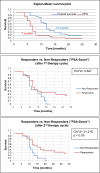
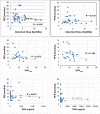
Results: 177Lu-PSMA-617 dosimetry after the first cycle indicated high tumor doses for skeletal (4.01 ± 2.64; range 1.10-13.00 Gy/GBq), lymph node (3.12 ± 2.07; range 0.70-8.70 Gy/GBq), and liver (2.97 ± 1.38; range 0.76-5.00 Gy/GBq) metastases whereas the dose for tissues/organs was acceptable in all patients for an intention-to-treat activity of 24 GBq. Any PSA decrease after the first cycle was found in 23/32 (72%), after the second cycle in 22/32 (69%), after the third cycle in 16/28 (57%), and after the fourth cycle in 8/18 (44%) patients. Post-therapy 24 h WB scintigraphy showed decreased tumor-to-background ratios in 24/32 (75%) after the first therapy cycle, after the second cycle in 17/29 (59%), and after the third cycle in 13/21 (62%) patients. The median PFS was 7 months and the median OS 12 months. In the group of PSA responders (n = 22) the median OS was 17 months versus 11 months in the group of non-responders (n = 10), p < 0.05. Decreasing SUVmax values were found for parotid (15.93 ± 6.23 versus 12.33 ± 4.07) and submandibular glands (17.65 ± 7.34 versus 13.12 ± 4.62) following treatment, along with transient (n = 6) or permanent (n = 2) xerostomia in 8/32 (25%) patients. In 3/32 patients, nephrotoxicity changed from Grade 2 to 3, whereas neither Grade 4 nephrotoxicity nor hematotoxicity was found. In most patients a good agreement was observed for the visual interpretation of the tracer accumulation between 24 h WB and PET/CT scans. However, no significance could be calculated for baseline-absorbed tumor doses and SUVmax values of tumor lesions. 5/32 (16%) patients showed a mixed response pattern, which resulted in disease progression over time.
Conclusion: Serial PSA measurements and post-therapy 24 h WB scintigraphy seems to allow a sufficiently accurate follow-up of 177Lu-PSMA-617-treated mCRPC patients whereas 68Ga-PSMA-11 PET/CT should be performed for patient selection and final response assessment.
Keywords: 177Lu-PSMA-617; 68Ga-PSMA-11; Dosimetry; Metastasized castration; Post-therapy whole-body scintigraphy; Theragnostic concept; resistant prostate cancer.
Conflict of interest statement
The authors declare that they have no conflicts of interest.
Figures


References
-
- Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, et al. New reponse evaluation criteria in solid tumours: revised RECIST guideline (version 1.1) Eur J Cancer. 2009;45:228–247. - PubMed
-
- Morigi JJ, Stricker PD, van Leeuwen PJ, et al. Prospective comparison of 18F-fluoromethylcholine versus 68Ga-PSMA PET/CT in prostate cancer patients who have rising PSA after curative treatment and are being considered for targeted therapy. J Nucl Med. 2015;56:1185–1190. - PubMed
-
- Seitz AK, Rauscher I, Haller B, Krönke M, Luther S, Heck MM, Horn T, Gschwend JE, Schwaiger M, Eiber M, Maurer T. Preliminary results on response assessment using 68Ga-HBED-CC-PSMA PET/CT in patients with metastatic prostate cancer undergoing docetaxel chemotherapy. Eur J Nucl Med Mol Imaging. 2018;45(4):602–612. - PubMed
-
- Uprimny C, Svirydenka A, Fritz J, Kroiss AS, Nilica B, Decristoforo C, Haubner R, von Guggenberg E, Buxbaum S, Horninger W, Virgolini IJ. Comparison of [68Ga]Ga-PSMA-11 PET/CT with [18F]NaF PET/CT in the evaluation of bone metastases in metastatic prostate cancer patients prior to radionuclide therapy. Eur J Nucl Med Mol Imaging. 2018;45(11):1873–1883. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

