Pharmacokinetics of Patisiran, the First Approved RNA Interference Therapy in Patients With Hereditary Transthyretin-Mediated Amyloidosis
- PMID: 31777097
- PMCID: PMC7187331
- DOI: 10.1002/jcph.1553
Pharmacokinetics of Patisiran, the First Approved RNA Interference Therapy in Patients With Hereditary Transthyretin-Mediated Amyloidosis
Abstract
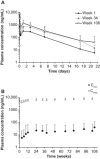
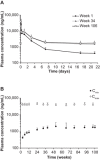
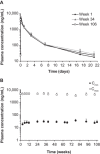
Hereditary transthyretin-mediated (hATTR) amyloidosis is a rare, inherited, progressively debilitating, and often fatal disease caused by deposition of mutated transthyretin (TTR) protein. Patisiran is an RNA interference therapeutic comprising a novel small interfering ribonucleic acid (ALN-18328) formulated with 2 novel lipid excipients, DLin-MC3-DMA and PEG2000 -C-DMG, in a lipid nanoparticle targeted to inhibit hepatic TTR synthesis. Here we report the pharmacokinetics (PK) of ALN-18328, DLin-MC3-DMA, and PEG2000 -C-DMG from a phase 2 multiple-ascending-dose study and its open-label extension (OLE) in patients with hATTR amyloidosis. Twenty-nine patients received 2 intravenous infusions of patisiran of 0.01, 0.05, 0.15, or 0.3 mg/kg at 3- or 4-week intervals; of these, 27 patients received 0.3 mg/kg once every 3 weeks over 24 months in the OLE study. Plasma PK profiles of ALN-18328 and DLin-MC3-DMA exhibited 2 phases, the first characterized by a short distribution half-life and the second by a minor peak and relatively long terminal elimination half-life. PK exposures to 3 analytes increased proportionally across the dose range of 0.01 to 0.3 mg/kg. For ALN-18328, mean terminal elimination half-life was 3.2 days, mean total clearance was 3.0 mL/h/kg, and urinary excretion was negligible. All 3 analytes exhibited stable PK profiles with chronic dosing over 2 years. The 2- to 3-fold plasma accumulation (AUCτ ) of ALN-18328 at steady state is attributable to the association of ALN-18328 with the cationic lipid DLin-MC3-DMA. There was no appreciable accumulation of PEG2000 -C-DMG.
Keywords: RNAi therapeutic; amyloidosis; lipid nanoparticle; patisiran; pharmacokinetics (PK); small interfering ribonucleic acid (siRNA).
© 2019 Alnylam Pharmaceuticals Inc. The Journal of Clinical Pharmacology published by Wiley Periodicals, Inc. on behalf of American College of Clinical Pharmacology.
Conflict of interest statement
The data in this article were obtained from the phase 2 multiple‐ascending‐dose study of patisiran and its open‐label extension, both funded by Alnylam Pharmaceuticals. Authors are full‐time employees of Alnylam Pharmaceuticals.
Figures
References
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

