Structure-based engineering of pH-dependent antibody binding for selective targeting of solid-tumor microenvironment
- PMID: 31777319
- PMCID: PMC6927761
- DOI: 10.1080/19420862.2019.1682866
Structure-based engineering of pH-dependent antibody binding for selective targeting of solid-tumor microenvironment
Abstract
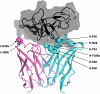
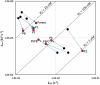
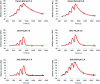
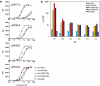
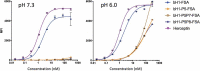
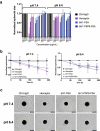
Recent development of monoclonal antibodies as mainstream anticancer agents demands further optimization of their safety for use in humans. Potent targeting and/or effector activities on normal tissues is an obvious toxicity concern. Optimization of specific tumor targeting could be achieved by taking advantage of the extracellular acidity of solid tumors relative to normal tissues. Here, we applied a structure-based computational approach to engineer anti-human epidermal growth factor receptor 2 (Her2) antibodies with selective binding in the acidic tumor microenvironment. We used an affinity maturation platform in which dual-pH histidine-scanning mutagenesis was implemented for pH selectivity optimization. Testing of a small set of designs for binding to the recombinant Her2 ectodomain led to the identification of antigen-binding fragment (Fab) variants with the desired pH-dependent binding behavior. Binding selectivity toward acidic pH was improved by as much as 25-fold relative to the parental bH1-Fab. In vitro experiments on cells expressing intact Her2 confirmed that designed variants formatted as IgG1/k full-size antibodies have high affinity and inhibit the growth of tumor spheroids at a level comparable to that of the benchmark anti-Her2 antibody trastuzumab (Herceptin®) at acidic pH, whereas these effects were significantly reduced at physiological pH. In contrast, both Herceptin and the parental bH1 antibody exhibited strong cell binding and growth inhibition irrespective of pH. This work demonstrates the feasibility of computational optimization of antibodies for selective targeting of the acidic environment such as that found in many solid tumors.
Keywords: Virtual histidine scanning; acidic pH selectivity; cell binding; spheroid growth; tumor targeting.
Figures
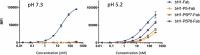
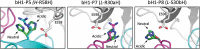
References
-
- Slaga D, Ellerman D, Lombana TN, Vij R, Li J, Hristopoulos M, Clark R, Johnston J, Shelton A, Mai E, et al. Avidity-based binding to Her2 results in selective killing of HER2-overexpressing cells by anti-HER2/CD3. Sci Transl Med. 2018;10:eaat5775. doi:10.1126/scitranslmed.aat5775. - PubMed
-
- Harms BD, Kearns JD, Su SV, Kohli N, Nielsen UB, Schoeberl B. Optimizing properties of antireceptor antibodies using kinetic computational models and experiments. Methods Enzymol. 2012;502:67–87. - PubMed
-
- Tannock IF, Rotin D. Acid pH in tumors and its potential for therapeutic exploitation. Cancer Res. 1989;49:4373–84. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous
