Effects of thyroid hormone on mitochondria and metabolism of human preimplantation embryos
- PMID: 31778245
- PMCID: PMC7064942
- DOI: 10.1002/stem.3129
Effects of thyroid hormone on mitochondria and metabolism of human preimplantation embryos
Abstract
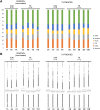
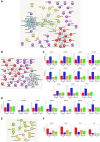
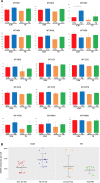
Thyroid hormones are regarded as the major controllers of metabolic rate and oxygen consumption in mammals. Although it has been demonstrated that thyroid hormone supplementation improves bovine embryo development in vitro, the cellular mechanisms underlying these effects are so far unknown. In this study, we investigated the role of thyroid hormone in development of human preimplantation embryos. Embryos were cultured in the presence or absence of 10-7 M triiodothyronine (T3) till blastocyst stage. Inner cell mass (ICM) and trophectoderm (TE) were separated mechanically and subjected to RNAseq or quantification of mitochondrial DNA copy number. Analyses were performed using DESeq (v1.16.0 on R v3.1.3), MeV4.9 and MitoMiner 4.0v2018 JUN platforms. We found that the exposure of human preimplantation embryos to T3 had a profound impact on nuclear gene transcription only in the cells of ICM (1178 regulated genes-10.5% of 11 196 expressed genes) and almost no effect on cells of TE (38 regulated genes-0.3% of expressed genes). The analyses suggest that T3 induces in ICM a shift in ribosome and oxidative phosphorylation activity, as the upregulated genes are contributing to the composition and organization of the respiratory chain and associated cofactors involved in mitoribosome assembly and stability. Furthermore, a number of genes affecting the citric acid cycle energy production have reduced expression. Our findings might explain why thyroid disorders in women have been associated with reduced fertility and adverse pregnancy outcome. Our data also raise a possibility that supplementation of culture media with T3 may improve outcomes for women undergoing in vitro fertilization.
Keywords: T3; embryo development; mitochondria; oxidative phosphorylation; thyroid hormone.
©2019 The Authors. Stem Cells published by Wiley Periodicals, Inc. on behalf of AlphaMed Press 2019.
Conflict of interest statement
The authors indicated no potential conflicts of interest.
Figures


References
-
- Krassas GE, Poppe K, Glinoer D. Thyroid function and human reproductive health. Endocr Rev. 2010;31:702‐755. - PubMed
-
- Wakim AN, Polizotto SL, Buffo MJ, Marrero MA, Burholt DR. Thyroid hormones in human follicular fluid and thyroid hormone receptors in human granulosa cells. Fertil Steril. 1993;59:1187‐1190. - PubMed
-
- Colicchia M, Campagnolo L, Baldini E, Ulisse S, Valensise H, Moretti C. Molecular basis of thyrotropin and thyroid hormone action during implantation and early development. Hum Reprod Update. 2014;20:884‐904. - PubMed
-
- Rho NY, Ashkar FA, Revay T, et al. De novo transcription of thyroid hormone receptors is essential for early bovine embryo development in vitro. Reprod Fertil Dev. 2018;30:779‐788. - PubMed
-
- Ashkar FA, Revay T, Rho N, et al. Thyroid hormones alter the transcriptome of in vitro‐produced bovine blastocysts. Zygote. 2016;24:266‐276. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

