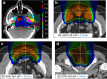
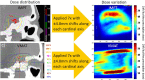
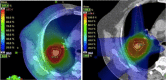
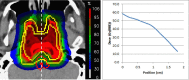
Myths and realities of range uncertainty
- PMID: 31778317
- PMCID: PMC7066970
- DOI: 10.1259/bjr.20190582
Myths and realities of range uncertainty
Abstract
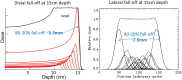
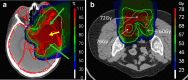
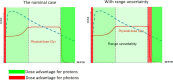
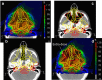
Range uncertainty is a much discussed topic in proton therapy. Although a very real aspect of proton therapy, its magnitude and consequences are sometimes misunderstood or overestimated. In this article, the sources and consequences of range uncertainty are reviewed, a number of myths associated with the effect discussed with the aim of putting range uncertainty into clinical context and attempting to de-bunk some of the more exaggerated claims made as to its consequences.
Figures