Conversion of Escherichia coli to Generate All Biomass Carbon from CO2
- PMID: 31778652
- PMCID: PMC6904909
- DOI: 10.1016/j.cell.2019.11.009
Conversion of Escherichia coli to Generate All Biomass Carbon from CO2
Abstract
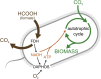
The living world is largely divided into autotrophs that convert CO2 into biomass and heterotrophs that consume organic compounds. In spite of widespread interest in renewable energy storage and more sustainable food production, the engineering of industrially relevant heterotrophic model organisms to use CO2 as their sole carbon source has so far remained an outstanding challenge. Here, we report the achievement of this transformation on laboratory timescales. We constructed and evolved Escherichia coli to produce all its biomass carbon from CO2. Reducing power and energy, but not carbon, are supplied via the one-carbon molecule formate, which can be produced electrochemically. Rubisco and phosphoribulokinase were co-expressed with formate dehydrogenase to enable CO2 fixation and reduction via the Calvin-Benson-Bassham cycle. Autotrophic growth was achieved following several months of continuous laboratory evolution in a chemostat under intensifying organic carbon limitation and confirmed via isotopic labeling.
Keywords: Escherichia coli; Rubisco; adaptive laboratory evolution; carbon fixation; metabolic rewiring; sustainability; synthetic autotrophy; synthetic biology.
Copyright © 2019 The Authors. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
We declare the following provisional patent related to the manuscript, “An Engineered Autotrophic
Figures







Comment in
-
E. coli bacteria engineered to eat carbon dioxide.Nature. 2019 Dec;576(7785):19-20. doi: 10.1038/d41586-019-03679-x. Nature. 2019. PMID: 31796902 No abstract available.
References
-
- Aigner H., Wilson R.H., Bracher A., Calisse L., Bhat J.Y., Hartl F.U., Hayer-Hartl M. Plant RuBisCo assembly in E. coli with five chloroplast chaperones including BSD2. Science. 2017;358:1272–1278. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

