The p53 inactivators pifithrin-μ and pifithrin-α mitigate TBI-induced neuronal damage through regulation of oxidative stress, neuroinflammation, autophagy and mitophagy
- PMID: 31778663
- PMCID: PMC7792017
- DOI: 10.1016/j.expneurol.2019.113135
The p53 inactivators pifithrin-μ and pifithrin-α mitigate TBI-induced neuronal damage through regulation of oxidative stress, neuroinflammation, autophagy and mitophagy
Abstract
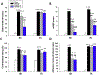
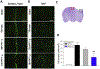
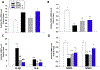
Traumatic brain injury (TBI) is one of the most common causes of death and disability worldwide. We investigated whether inhibition of p53 using pifithrin (PFT)-α or PFT-μ provides neuroprotective effects via p53 transcriptional dependent or -independent mechanisms, respectively. Sprague Dawley rats were subjected to controlled cortical impact TBI followed by the administration of PFTα or PFT-μ (2 mg/kg, i.v.) at 5 h after TBI. Brain contusion volume, as well as sensory and motor functions were evaluated at 24 h after TBI. TBI-induced impairments were mitigated by both PFT-α and PFT-μ. Fluoro-Jade C staining was used to label degenerating neurons within the TBI-induced cortical contusion region that, together with Annexin V positive neurons, were reduced by PFT-μ. Double immunofluorescence staining similarly demonstrated that PFT-μ significantly increased HO-1 positive neurons and mRNA expression in the cortical contusion region as well as decreased numbers of 4-hydroxynonenal (4HNE)-positive cells. Levels of mRNA encoding for p53, autophagy, mitophagy, anti-oxidant, anti-inflammatory related genes and proteins were measured by RT-qPCR and immunohistochemical staining, respectively. PFT-α, but not PFT-μ, significantly lowered p53 mRNA expression. Both PFT-α and PFT-μ lowered TBI-induced pro-inflammatory cytokines (IL-1β and IL-6) mRNA levels as well as TBI-induced autophagic marker localization (LC3 and p62). Finally, treatment with PFT-μ mitigated TBI-induced declines in mRNA levels of PINK-1 and SOD2. Our data suggest that both PFT-μ and PFT-α provide neuroprotective actions through regulation of oxidative stress, neuroinflammation, autophagy, and mitophagy mechanisms, and that PFT-μ, in particular, holds promise as a TBI treatment strategy.
Keywords: Autophagy; Mitophagy; Neuroinflammation; PFT-α; PFT-μ; Pifithrin analogs; Traumatic brain injury (TBI); p53.
Copyright © 2019. Published by Elsevier Inc.
Conflict of interest statement
Declaration of Competing Interest
The authors declare no conflicts of Interest.
Figures





References
-
- Arango D, Corner GA, Wadler S, Catalano PJ, 2001. Augenlicht, L.H. C-myc/p53 interaction determines sensitivity of human colon carcinoma cells to 5-fluorouracil in vitro and in vivo. Cancer Res. 61, 4910–4915. - PubMed
-
- Barkhoudarian G, Hovda DA, Giza CC, 2011. The molecular pathophysiology of concussive brain injury. Clin. Sports Med 30, 33–48. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

