Two Potential Clinical Applications of Origami-Based Paper Devices
- PMID: 31779180
- PMCID: PMC6963803
- DOI: 10.3390/diagnostics9040203
Two Potential Clinical Applications of Origami-Based Paper Devices
Abstract
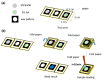
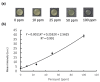
Detecting small amounts of analyte in clinical practice is challenging because of deficiencies in specimen sample availability and unsuitable sampling environments that prevent reliable sampling. Paper-based analytical devices (PADs) have successfully been used to detect ultralow amounts of analyte, and origami-based PADs (O-PADs) offer advantages that may boost the overall potential of PADs in general. In this study, we investigated two potential clinical applications for O-PADs. The first O-PAD we investigated was an origami-based enzyme-linked immunosorbent assay (ELISA) system designed to detect different concentrations of rabbit IgG. This device was designed with four wing structures, each of which acted as a reagent loading zone for pre-loading ELISA reagents, and a central test sample loading zone. Because this device has a low limit of detection (LOD), it may be suitable for detecting IgG levels in tears from patients with a suspected viral infection (such as herpes simplex virus (HSV)). The second O-PAD we investigated was designed to detect paraquat levels to determine potential poisoning. To use this device, we sequentially folded each of two separate reagent zones, one preloaded with NaOH and one preloaded with ascorbic acid (AA), over the central test zone, and added 8 µL of sample that then flowed through each reagent zone and onto the central test zone. The device was then unfolded to read the results on the test zone. The three folded layers of paper provided a moist environment not achievable with conventional paper-based ELISA. Both O-PADs were convenient to use because reagents were preloaded, and results could be observed and analyzed with image analysis software. O-PADs expand the testing capacity of simpler PADs while leveraging their characteristic advantages of convenience, cost, and ease of use, particularly for point-of-care diagnosis.
Keywords: IgG; origami ELISA; origami-based paper analytic device; paraquat.
Conflict of interest statement
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Figures



References
-
- Hsu M.Y., Hung Y.C., Hwang D.K., Lin S.C., Lin K.H., Wang C.Y., Choi H.Y., Wang Y.P., Cheng C.M. Detection of aqueous VEGF concentrations before and after intravitreal injection of anti-VEGF antibody using low-volume sampling paper-based ELISA. Sci. Rep. 2016;6:34631. doi: 10.1038/srep34631. - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources

