The Aquilegia genome reveals a hybrid origin of core eudicots
- PMID: 31779695
- PMCID: PMC6883705
- DOI: 10.1186/s13059-019-1888-8
The Aquilegia genome reveals a hybrid origin of core eudicots
Abstract
Background: Whole-genome duplications (WGDs) have dominated the evolutionary history of plants. One consequence of WGD is a dramatic restructuring of the genome as it undergoes diploidization, a process under which deletions and rearrangements of various sizes scramble the genetic material, leading to a repacking of the genome and eventual return to diploidy. Here, we investigate the history of WGD in the columbine genus Aquilegia, a basal eudicot, and use it to illuminate the origins of the core eudicots.
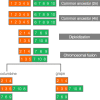
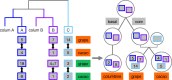
Results: Within-genome synteny confirms that columbines are ancient tetraploids, and comparison with the grape genome reveals that this tetraploidy appears to be shared with the core eudicots. Thus, the ancient gamma hexaploidy found in all core eudicots must have involved a two-step process: first, tetraploidy in the ancestry of all eudicots, then hexaploidy in the ancestry of core eudicots. Furthermore, the precise pattern of synteny sharing suggests that the latter involved allopolyploidization and that core eudicots thus have a hybrid origin.
Conclusions: Novel analyses of synteny sharing together with the well-preserved structure of the columbine genome reveal that the gamma hexaploidy at the root of core eudicots is likely a result of hybridization between a tetraploid and a diploid species.
Conflict of interest statement
The authors declare no competing interests.
Figures



Comment in
-
A reappraisal of the phylogenetic placement of the Aquilegia whole-genome duplication.Genome Biol. 2020 Dec 8;21(1):295. doi: 10.1186/s13059-020-02212-y. Genome Biol. 2020. PMID: 33292440 Free PMC article.
References
-
- Leitch IJ, Bennett MD. Genome downsizing in polyploid plants. Biol J Linn Soc Lond. Oxford University Press. 2004;82:651–663.
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

