Intraspinal Plasticity Associated With the Development of Autonomic Dysreflexia After Complete Spinal Cord Injury
- PMID: 31780900
- PMCID: PMC6856770
- DOI: 10.3389/fncel.2019.00505
Intraspinal Plasticity Associated With the Development of Autonomic Dysreflexia After Complete Spinal Cord Injury
Abstract
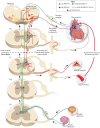
Traumatic spinal cord injury (SCI) leads to disruption of sensory, motor and autonomic function, and triggers structural, physiological and biochemical changes that cause reorganization of existing circuits that affect functional recovery. Propriospinal neurons (PN) appear to be very plastic within the inhibitory microenvironment of the injured spinal cord by forming compensatory circuits that aid in relaying information across the lesion site and, thus, are being investigated for their potential to promote locomotor recovery after experimental SCI. Yet the role of PN plasticity in autonomic dysfunction is not well characterized, notably, the disruption of supraspinal modulatory signals to spinal sympathetic neurons after SCI at the sixth thoracic spinal segment or above resulting in autonomic dysreflexia (AD). This condition is characterized by unmodulated sympathetic reflexes triggering sporadic hypertension associated with baroreflex mediated bradycardia in response to noxious yet unperceived stimuli below the injury to reduce blood pressure. AD is frequently triggered by pelvic visceral distension (bowel and bladder), and there are documented structural relationships between injury-induced sprouting of pelvic visceral afferent C-fibers. Their excitation of lumbosacral PN, in turn, sprout and relay noxious visceral sensory stimuli to rostral disinhibited thoracic sympathetic preganglionic neurons (SPN) that manifest hypertension. Herein, we review evidence for maladaptive plasticity of PN in neural circuits mediating heightened sympathetic reflexes after complete high thoracic SCI that manifest cardiovascular dysfunction, as well as contemporary research methodologies being employed to unveil the precise contribution of PN plasticity to the pathophysiology underlying AD development.
Keywords: cardiovascular dysfunction; hypertension; interneuron; propriospinal; sympathetic preganglionic neurons.
Copyright © 2019 Michael, Patel and Rabchevsky.
Figures
Similar articles
-
Segmental organization of spinal reflexes mediating autonomic dysreflexia after spinal cord injury.Prog Brain Res. 2006;152:265-74. doi: 10.1016/S0079-6123(05)52017-X. Prog Brain Res. 2006. PMID: 16198706 Free PMC article. Review.
-
Autonomic dysreflexia after spinal cord injury: Systemic pathophysiology and methods of management.Auton Neurosci. 2018 Jan;209:59-70. doi: 10.1016/j.autneu.2017.05.002. Epub 2017 May 8. Auton Neurosci. 2018. PMID: 28506502 Free PMC article. Review.
-
Plasticity of lumbosacral propriospinal neurons is associated with the development of autonomic dysreflexia after thoracic spinal cord transection.J Comp Neurol. 2008 Aug 1;509(4):382-99. doi: 10.1002/cne.21771. J Comp Neurol. 2008. PMID: 18512692 Free PMC article.
-
Genetic manipulation of intraspinal plasticity after spinal cord injury alters the severity of autonomic dysreflexia.J Neurosci. 2006 Mar 15;26(11):2923-32. doi: 10.1523/JNEUROSCI.4390-05.2006. J Neurosci. 2006. PMID: 16540569 Free PMC article.
-
Paradoxical effects of continuous high dose gabapentin treatment on autonomic dysreflexia after complete spinal cord injury.Exp Neurol. 2020 Jan;323:113083. doi: 10.1016/j.expneurol.2019.113083. Epub 2019 Oct 31. Exp Neurol. 2020. PMID: 31678138 Free PMC article.
Cited by
-
The role of arousal and motivation in emotional conflict resolution: Implications for spinal cord injury.Front Hum Neurosci. 2022 Oct 5;16:927622. doi: 10.3389/fnhum.2022.927622. eCollection 2022. Front Hum Neurosci. 2022. PMID: 36277056 Free PMC article.
-
Phylogenetic view of the compensatory mechanisms in motor and sensory systems after neuronal injury.Curr Res Neurobiol. 2022 Oct 17;3:100058. doi: 10.1016/j.crneur.2022.100058. eCollection 2022. Curr Res Neurobiol. 2022. PMID: 36304591 Free PMC article. Review.
-
Role of Descending Serotonergic Fibers in the Development of Pathophysiology after Spinal Cord Injury (SCI): Contribution to Chronic Pain, Spasticity, and Autonomic Dysreflexia.Biology (Basel). 2022 Feb 1;11(2):234. doi: 10.3390/biology11020234. Biology (Basel). 2022. PMID: 35205100 Free PMC article. Review.
-
Evaluation of the Cardiometabolic Disorders after Spinal Cord Injury in Mice.Biology (Basel). 2022 Mar 24;11(4):495. doi: 10.3390/biology11040495. Biology (Basel). 2022. PMID: 35453695 Free PMC article.
-
Propriospinal myoclonus following cervical spinal cord injury: a case report and mechanistic insights.J Neurol. 2025 Jan 15;272(2):126. doi: 10.1007/s00415-024-12880-6. J Neurol. 2025. PMID: 39812827 Free PMC article. No abstract available.
References
-
- Alstermark B., Lindstrom S., Lundberg A., Sybirska E. (1981). Integration in descending motor pathways controlling the forelimb in the cat: 8. Ascending projection to the lateral reticular nucleus from C3–C4 propriospinal also projecting to forelimb motoneurones. Exp. Brain Res. 42, 282–298. 10.1007/bf00237495 - DOI - PubMed
-
- Alstermark B., Lundberg A., Sasaki S. (1984). Integration in descending motor pathways controlling the forelimb in the cat. 12. Interneurones which may mediate descending feed-forward inhibition and feed-back inhibition from the forelimb to C3–C4 propriospinal neurones. Exp. Brain Res. 56, 308–322. 10.1007/bf00236286 - DOI - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources

