Synergy of Glutamatergic and Cholinergic Modulation Induces Plateau Potentials in Hippocampal OLM Interneurons
- PMID: 31780902
- PMCID: PMC6861217
- DOI: 10.3389/fncel.2019.00508
Synergy of Glutamatergic and Cholinergic Modulation Induces Plateau Potentials in Hippocampal OLM Interneurons
Abstract
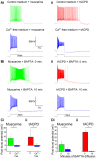
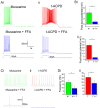
Oriens-lacunosum moleculare (OLM) cells are hippocampal inhibitory interneurons that are implicated in the regulation of information flow in the CA1 circuit, inhibiting cortical inputs to distal pyramidal cell dendrites, whilst disinhibiting CA3 inputs to pyramidal cells. OLM cells express metabotropic cholinergic (mAChR) and glutamatergic (mGluR) receptors, so modulation of these cells via these receptors may contribute to switching between functional modes of the hippocampus. Using a transgenic mouse line to identify OLM cells, we found that both mAChR and mGluR activation caused the cells to exhibit long-lasting depolarizing plateau potentials following evoked spike trains. Both mAChR- and mGluR-induced plateau potentials were eliminated by blocking transient receptor potential (TRP) channels, and were dependent on intracellular calcium concentration and calcium entry. Pharmacological tests indicated that Group I mGluRs are responsible for the glutamatergic induction of plateaus. There was also a pronounced synergy between the cholinergic and glutamatergic modulation, plateau potentials being generated by agonists applied together at concentrations too low to elicit any change when applied individually. This synergy could enable OLM cells to function as coincidence detectors of different neuromodulatory systems, leading to their enhanced and prolonged activation and a functional change in information flow within the hippocampus.
Keywords: OLM cells; TRP channel; interneuron; mAChR; mGluR; plateau potential; synergy.
Copyright © 2019 Hagger-Vaughan and Storm.
Figures




References
-
- Bocchio M., Lukacs I. P., Stacey R., Plaha P., Apostolopoulos V., Livermore L., et al. (2019). Group II metabotropic glutamate receptors mediate presynaptic inhibition of excitatory transmission in pyramidal neurons of the human cerebral cortex. Front. Cell. Neurosci. 12:508. 10.3389/fncel.2018.00508 - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

