Ovarian Hormones Mediate Changes in Adaptive Choice and Motivation in Female Rats
- PMID: 31780908
- PMCID: PMC6861187
- DOI: 10.3389/fnbeh.2019.00250
Ovarian Hormones Mediate Changes in Adaptive Choice and Motivation in Female Rats
Abstract
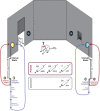
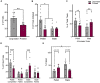
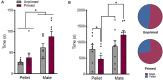
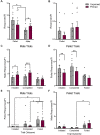
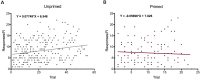
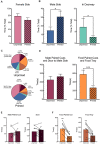
In female rodents, sexual receptivity is coordinated with cyclic changes in the release of gonadal hormones. Increases in estradiol (E) and progesterone (P) during proestrus and estrus not only induce ovulation but also modulate behaviors that increase the likelihood that the female will find a mate and reproduce. This includes changes in receptive behaviors, such as lordosis, as well as changes in appetitive or proceptive behaviors, including motivation. Interestingly, the direction of these changes in motivation is dependent on the type of reward that is being pursued. While induction of sexual receptivity by E and P increases motivation for access to a male, motivation for a palatable food reward is decreased. These concurrent changes may facilitate adaptive choice across the estrous cycle; females bias their choice for sex when fertilization is most likely to occur, but for food when copulation is unlikely to result in impregnation. In order to test this hypothesis, we developed a novel paradigm to measure the motivated choice between a palatable food reward and access to a male conspecific. Ovariectomized, hormone primed females were trained to operantly respond for both food and sex on a fixed interval (FI) schedule. After training, unprimed and primed females were tested in a chamber that allows them to choose between food and sex while still requiring responding on the FI schedule for reach reward. From this we can not only determine the impact of hormone priming on female choice for food or sex, but also how this is reflected by changes in motivation for each specific reward, as measured by the average number of responses made during each fixed interval. Induction of sexual receptivity by hormone priming biases choice toward sex over food and this change is accompanied by an increase in motivation for sex but a decrease in motivation for food. This work provides evidence in support of a novel framework for understanding how the release of ovarian hormones over the course of the estrous cycle modulates adaptive behavioral choice in females by directly assessing motivation via operant responding when multiple rewards are available.
Keywords: feeding; females; motivation; ovarian hormones; sexual behavior.
Copyright © 2019 Yoest, Cummings and Becker.
Figures
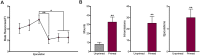
Similar articles
-
Action of luteinizing hormone-releasing factor (lrf) in the initiation of lordosis behavior in the estrone-primed ovariectomized female rat.Neuroendocrinology. 1975;17(4):309-18. doi: 10.1159/000122369. Neuroendocrinology. 1975. PMID: 806824
-
Food restriction-induced changes in motivation differ with stages of the estrous cycle and are closely linked to RFamide-related peptide-3 but not kisspeptin in Syrian hamsters.Physiol Behav. 2018 Jun 1;190:43-60. doi: 10.1016/j.physbeh.2017.06.009. Epub 2017 Jun 15. Physiol Behav. 2018. PMID: 28624479 Free PMC article.
-
The neurosteroids, progesterone and 3alpha,5alpha-THP, enhance sexual motivation, receptivity, and proceptivity in female rats.Brain Res. 1998 Oct 12;808(1):72-83. doi: 10.1016/s0006-8993(98)00764-1. Brain Res. 1998. PMID: 9795145
-
Estradiol, dopamine and motivation.Cent Nerv Syst Agents Med Chem. 2014;14(2):83-9. doi: 10.2174/1871524914666141226103135. Cent Nerv Syst Agents Med Chem. 2014. PMID: 25540977 Free PMC article. Review.
-
"What a Girl Wants": What Can We Learn From Animal Models of Female Sexual Motivation?Front Behav Neurosci. 2019 Sep 20;13:216. doi: 10.3389/fnbeh.2019.00216. eCollection 2019. Front Behav Neurosci. 2019. PMID: 31619975 Free PMC article. Review.
Cited by
-
Neurobiology of Pathogen Avoidance and Mate Choice: Current and Future Directions.Animals (Basel). 2024 Jan 17;14(2):296. doi: 10.3390/ani14020296. Animals (Basel). 2024. PMID: 38254465 Free PMC article. Review.
-
Beyond sex and aggression: testosterone rapidly matches behavioural responses to social context and tries to predict the future.Proc Biol Sci. 2022 Jun 8;289(1976):20220453. doi: 10.1098/rspb.2022.0453. Epub 2022 Jun 8. Proc Biol Sci. 2022. PMID: 35673866 Free PMC article.
-
Hypothalamic AgRP neurons regulate the hyperphagia of lactation.Mol Metab. 2024 Aug;86:101975. doi: 10.1016/j.molmet.2024.101975. Epub 2024 Jun 24. Mol Metab. 2024. PMID: 38925247 Free PMC article.
-
Effects of sex and estrous cycle on action-outcome contingencies.Behav Brain Res. 2025 Feb 4;477:115317. doi: 10.1016/j.bbr.2024.115317. Epub 2024 Oct 28. Behav Brain Res. 2025. PMID: 39490537
-
Activation of G protein-coupled estradiol receptor 1 in the dorsolateral striatum enhances motivation for cocaine and drug-induced reinstatement in female but not male rats.Biol Sex Differ. 2021 Aug 14;12(1):46. doi: 10.1186/s13293-021-00389-w. Biol Sex Differ. 2021. PMID: 34391470 Free PMC article.
References
-
- Adler N. T., McClintock M. K. (1978). The role of the female during copulation in wild and domestic norway rats (Rattus norvegicus). Behaviour 67, 67–95. 10.1163/156853978X00260 - DOI
Grants and funding
LinkOut - more resources
Full Text Sources

