Reactivation of the Unconditioned Stimulus Inhibits the Return of Fear Independent of Cortisol
- PMID: 31780910
- PMCID: PMC6861211
- DOI: 10.3389/fnbeh.2019.00254
Reactivation of the Unconditioned Stimulus Inhibits the Return of Fear Independent of Cortisol
Abstract
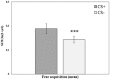
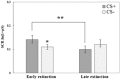
Reconsolidation is the post-retrieval stabilization of memories, a time-limited process during which reactivated (i.e., retrieved) memories can be updated with new information, become stronger or weaker, depending on the specific treatment. We have previously shown that the stress hormone cortisol has an enhancing effect on the reconsolidation of fear memories in men. This effect was specific, i.e., limited to the conditioned stimulus (CS) that was reactivated, and did not generalize to other previously reinforced, but not reactivated CS. Based on these results, we suggested that cortisol plays a critical role in the continuous strengthening of reactivated emotional memories, contributing to their persistence and robustness. In the current study, we aimed to achieve a more generalized reconsolidation enhancement using an alternative reactivation method, i.e., by a low-intensity unconditioned stimulus (UCS) presentation instead of the more common unreinforced CS presentation. In previous studies, UCS reactivation was shown to lead to a more generalized reconsolidation effect. Therefore, we hypothesized that the combination of cortisol treatment and UCS reactivation would lead to an enhanced fear memory reconsolidation, which would generalize from previously reinforced CS to stimuli that resemble it. We tested 75 men in a 3-day fear conditioning paradigm: fear acquisition training on day 1; UCS reactivation/no reactivation and pharmacological treatment (20 mg hydrocortisone/placebo) on day 2; extinction training, reinstatement and test (of original and modified stimuli) on day 3. In contrast to our hypothesis, UCS reactivation prevented the return of fear [observed in skin conductance responses (SCR)] regardless of the pharmacological manipulation: while reinstatement to the original CS was found in the no-reactivation group, both reactivation groups (cortisol and placebo) showed no reinstatement. As the only methodological difference between our previous study and the current one was the reactivation method, we focus on UCS reactivation as the main explanation for these unexpected findings. We suggest that the robust prediction error generated by the UCS reactivation method (as opposed to CS reactivation), combined with the lower UCS intensity, has by itself weakened the emotional value of the UCS, thus preventing the return of fear to the CS that was associated with it. We call for future research to support these findings and to examine the potential of this reactivation method, or variations thereof, as a tool for therapeutic use.
Keywords: extinction; fear conditioning; glucocorticoids; reconsolidation; reinstatement; retrieval.
Copyright © 2019 Meir Drexler, Merz, Lissek, Tegenthoff and Wolf.
Figures

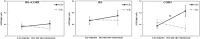

Similar articles
-
Effects of Cortisol on Reconsolidation of Reactivated Fear Memories.Neuropsychopharmacology. 2015 Dec;40(13):3036-43. doi: 10.1038/npp.2015.160. Epub 2015 Jun 10. Neuropsychopharmacology. 2015. PMID: 26058664 Free PMC article.
-
Stress disrupts the reconsolidation of fear memories in men.Psychoneuroendocrinology. 2017 Mar;77:95-104. doi: 10.1016/j.psyneuen.2016.11.027. Epub 2016 Nov 25. Psychoneuroendocrinology. 2017. PMID: 28024275 Clinical Trial.
-
Effect of Selective Inhibition of Reactivated Nicotine-Associated Memories With Propranolol on Nicotine Craving.JAMA Psychiatry. 2017 Mar 1;74(3):224-232. doi: 10.1001/jamapsychiatry.2016.3907. JAMA Psychiatry. 2017. PMID: 28146250 Free PMC article.
-
Human reconsolidation: a reactivation and update.Brain Res Bull. 2014 Jun;105:70-82. doi: 10.1016/j.brainresbull.2013.12.010. Epub 2014 Jan 4. Brain Res Bull. 2014. PMID: 24397965 Review.
-
How stress hormones shape memories of fear and anxiety in humans.Neurosci Biobehav Rev. 2022 Nov;142:104901. doi: 10.1016/j.neubiorev.2022.104901. Epub 2022 Oct 10. Neurosci Biobehav Rev. 2022. PMID: 36228925 Review.
Cited by
-
Navigating the garden of forking paths for data exclusions in fear conditioning research.Elife. 2019 Dec 16;8:e52465. doi: 10.7554/eLife.52465. Elife. 2019. PMID: 31841112 Free PMC article.
-
Propranolol-induced inhibition of unconditioned stimulus-reactivated fear memory prevents the return of fear in humans.Transl Psychiatry. 2020 Oct 13;10(1):345. doi: 10.1038/s41398-020-01023-w. Transl Psychiatry. 2020. PMID: 33051441 Free PMC article.
-
Targeting Human Glucocorticoid Receptors in Fear Learning: A Multiscale Integrated Approach to Study Functional Connectivity.Int J Mol Sci. 2024 Jan 10;25(2):864. doi: 10.3390/ijms25020864. Int J Mol Sci. 2024. PMID: 38255937 Free PMC article. Review.
References
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

