Cytoprotective Effects of Dinitrosyl Iron Complexes on Viability of Human Fibroblasts and Cardiomyocytes
- PMID: 31780929
- PMCID: PMC6859909
- DOI: 10.3389/fphar.2019.01277
Cytoprotective Effects of Dinitrosyl Iron Complexes on Viability of Human Fibroblasts and Cardiomyocytes
Abstract
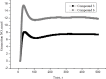
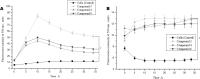
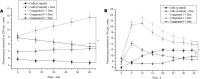
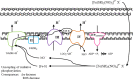
Nitric oxide (NO) is an important signaling molecule that plays a key role in maintaining vascular homeostasis. Dinitrosyl iron complexes (DNICs) generating NO are widely used to treat cardiovascular diseases. However, the involvement of DNICs in the metabolic processes of the cell, their protective properties in doxorubicin-induced toxicity remain to be clarified. Here, we found that novel class of mononuclear DNICs with functional sulfur-containing ligands enhanced the cell viability of human lung fibroblasts and rat cardiomyocytes. Moreover, DNICs demonstrated remarkable protection against doxorubicin-induced toxicity in fibroblasts and in rat cardiomyocytes (H9c2 cells). Data revealed that the DNICs compounds modulate the mitochondria function by decreasing the mitochondrial membrane potential (ΔΨm). Results of flow cytometry showed that DNICs were not affected the proliferation, growth of fibroblasts. In addition, this study showed that DNICs did not affect glutathione levels and the formation of reactive oxygen species in cells. Moreover, results indicated that DNICs maintained the ATP equilibrium in cells. Taken together, these findings show that DNICs have protective properties in vitro. It was further suggested that DNICs may be uncouplers of oxidative phosphorylation in mitochondria and protective mechanism is mainly provided by the leakage of excess charge through the mitochondrial membrane. It is assumed that the DNICs have the therapeutic potential for treating cardiovascular diseases and for decreasing of chemotherapy-induced cardiotoxicity in cancer survivors.
Keywords: cell viability; dinitrosyl iron complexes; donors nitric oxide; heart disease; membrane potential.
Copyright © 2019 Akentieva, Sanina, Gizatullin, Shkondina, Prikhodchenko, Shram, Zhelev and Aldoshin.
Figures







Similar articles
-
Effect of Dinitrosyl Iron Complexes (NO Donors) on the Metabolic Processes in Human Fibroblasts.Dokl Biochem Biophys. 2018 Nov;483(1):337-340. doi: 10.1134/S1607672918060133. Epub 2019 Jan 3. Dokl Biochem Biophys. 2018. PMID: 30607734
-
Polynuclear water-soluble dinitrosyl iron complexes with cysteine or glutathione ligands: electron paramagnetic resonance and optical studies.Nitric Oxide. 2010 Sep 15;23(2):136-49. doi: 10.1016/j.niox.2010.05.285. Epub 2010 May 27. Nitric Oxide. 2010. PMID: 20553936
-
Detection and determination of the {Fe(NO)(2)} core vibrational features in dinitrosyl-iron complexes from experiment, normal coordinate analysis, and density functional theory: an avenue for probing the nitric oxide oxidation state.J Phys Chem B. 2007 Mar 8;111(9):2335-46. doi: 10.1021/jp066964f. Epub 2007 Feb 13. J Phys Chem B. 2007. PMID: 17295535
-
Synthetic methodology for preparation of dinitrosyl iron complexes.J Biol Inorg Chem. 2019 Jun;24(4):495-515. doi: 10.1007/s00775-019-01668-z. Epub 2019 May 20. J Biol Inorg Chem. 2019. PMID: 31111233 Review.
-
[Dinitrosyl iron complexes--structure and biological functions].Postepy Biochem. 2010;56(3):298-304. Postepy Biochem. 2010. PMID: 21117318 Review. Polish.
Cited by
-
Effects of Nitrosyl Iron Complexes with Thiol, Phosphate, and Thiosulfate Ligands on Hemoglobin.Int J Mol Sci. 2024 Jun 29;25(13):7194. doi: 10.3390/ijms25137194. Int J Mol Sci. 2024. PMID: 39000305 Free PMC article.
-
Role of GSH and Iron-Sulfur Glutaredoxins in Iron Metabolism-Review.Molecules. 2020 Aug 25;25(17):3860. doi: 10.3390/molecules25173860. Molecules. 2020. PMID: 32854270 Free PMC article. Review.
-
Inflammation, dysregulated iron metabolism, and cardiovascular disease.Front Aging. 2023 Feb 3;4:1124178. doi: 10.3389/fragi.2023.1124178. eCollection 2023. Front Aging. 2023. PMID: 36816471 Free PMC article. Review.
-
Histidine-Bound Dinitrosyl Iron Complexes: Antioxidant and Antiradical Properties.Int J Mol Sci. 2023 Dec 7;24(24):17236. doi: 10.3390/ijms242417236. Int J Mol Sci. 2023. PMID: 38139065 Free PMC article.
-
Positive (Regulatory) and Negative (Cytotoxic) Effects of Dinitrosyl Iron Complexes on Living Organisms.Biochemistry (Mosc). 2022 Nov;87(11):1367-1386. doi: 10.1134/S0006297922110153. Biochemistry (Mosc). 2022. PMID: 36509730 Free PMC article. Review.
References
-
- Aldoshin S. M., Sanina N. A., Davydov M. I., Chazov E. I. (2016). A new class of nitrogen oxide donors. Herald Russian Acad. Sci. 86 (3), 495–500. 10.1134/S1019331616030096 - DOI
LinkOut - more resources
Full Text Sources

