Stem Cells, Self-Renewal, and Lineage Commitment in the Endocrine System
- PMID: 31781041
- PMCID: PMC6856655
- DOI: 10.3389/fendo.2019.00772
Stem Cells, Self-Renewal, and Lineage Commitment in the Endocrine System
Abstract
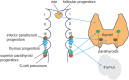
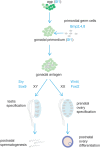
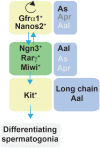
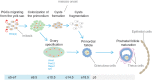
The endocrine system coordinates a wide array of body functions mainly through secretion of hormones and their actions on target tissues. Over the last decades, a collective effort between developmental biologists, geneticists, and stem cell biologists has generated a wealth of knowledge related to the contribution of stem/progenitor cells to both organogenesis and self-renewal of endocrine organs. This review provides an up-to-date and comprehensive overview of the role of tissue stem cells in the development and self-renewal of endocrine organs. Pathways governing crucial steps in both development and stemness maintenance, and that are known to be frequently altered in a wide array of endocrine disorders, including cancer, are also described. Crucially, this plethora of information is being channeled into the development of potential new cell-based treatment modalities for endocrine-related illnesses, some of which have made it through clinical trials.
Keywords: development; plasticity; regenerative medicine; self-renewal; stem cells.
Copyright © 2019 Mariniello, Ruiz-Babot, McGaugh, Nicholson, Gualtieri, Gaston-Massuet, Nostro and Guasti.
Figures

Similar articles
-
The regenerative nidi of the locust midgut as a model to study epithelial cell differentiation from stem cells.J Exp Biol. 2006 Jun;209(Pt 11):2215-23. doi: 10.1242/jeb.02249. J Exp Biol. 2006. PMID: 16709922
-
Potential renal stem/progenitor cells identified by in vivo lineage tracing.Am J Physiol Renal Physiol. 2022 Apr 1;322(4):F379-F391. doi: 10.1152/ajprenal.00326.2021. Epub 2022 Jan 31. Am J Physiol Renal Physiol. 2022. PMID: 35100814 Free PMC article. Review.
-
Nephron progenitor commitment is a stochastic process influenced by cell migration.Elife. 2019 Jan 24;8:e41156. doi: 10.7554/eLife.41156. Elife. 2019. PMID: 30676318 Free PMC article.
-
The involvement of bioactive factors in the self-renewal and stemness maintenance of spermatogonial stem cells.Mol Cell Biochem. 2021 Apr;476(4):1813-1823. doi: 10.1007/s11010-020-04028-7. Epub 2021 Jan 18. Mol Cell Biochem. 2021. PMID: 33459979 Review.
-
Molecular signatures of self-renewal, differentiation, and lineage choice in multipotential hemopoietic progenitor cells in vitro.Mol Cell Biol. 2004 Jan;24(2):741-56. doi: 10.1128/MCB.24.2.741-756.2004. Mol Cell Biol. 2004. PMID: 14701746 Free PMC article.
Cited by
-
Distribution, contribution and regulation of nestin+ cells.J Adv Res. 2024 Jul;61:47-63. doi: 10.1016/j.jare.2023.08.013. Epub 2023 Aug 28. J Adv Res. 2024. PMID: 37648021 Free PMC article. Review.
-
Molecular Mechanisms of Pituitary Cell Plasticity.Front Endocrinol (Lausanne). 2020 Sep 10;11:656. doi: 10.3389/fendo.2020.00656. eCollection 2020. Front Endocrinol (Lausanne). 2020. PMID: 33013715 Free PMC article. Review.
-
Xenogenic Engraftment of Human-Induced Pluripotent Stem Cell-Derived Pancreatic Islet Cells in an Immunosuppressive Diabetic Göttingen Mini-Pig Model.Cell Transplant. 2024 Jan-Dec;33:9636897241288932. doi: 10.1177/09636897241288932. Cell Transplant. 2024. PMID: 39401129 Free PMC article.
-
Experimental Evidence and Clinical Implications of Pituitary Adenoma Stem Cells.Front Endocrinol (Lausanne). 2020 Feb 20;11:54. doi: 10.3389/fendo.2020.00054. eCollection 2020. Front Endocrinol (Lausanne). 2020. PMID: 32153500 Free PMC article. Review.
-
Harnessing gut cells for functional insulin production: Strategies and challenges.Biotechnol Notes. 2022 Dec 9;4:7-13. doi: 10.1016/j.biotno.2022.11.005. eCollection 2023. Biotechnol Notes. 2022. PMID: 39416909 Free PMC article. Review.
References
-
- Raetzman LT, Ward R, Camper SA. Lhx4 and Prop1 are required for cell survival and expansion of the pituitary primordia. Development. (2002) 129:4229–39. - PubMed
Publication types
LinkOut - more resources
Full Text Sources

