Grafting Cucumber Onto Pumpkin Induced Early Stomatal Closure by Increasing ABA Sensitivity Under Salinity Conditions
- PMID: 31781131
- PMCID: PMC6859870
- DOI: 10.3389/fpls.2019.01290
Grafting Cucumber Onto Pumpkin Induced Early Stomatal Closure by Increasing ABA Sensitivity Under Salinity Conditions
Abstract
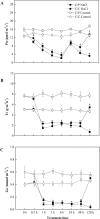
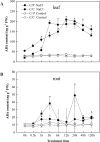
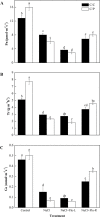
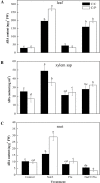
During early periods of salt stress, reduced stomatal opening can prevent water loss and wilting. Abscisic acid (ABA) signal plays an important role in this process. Here, we show that cucumber grafted onto pumpkin exhibits rapid stomatal closure, which helps plants to adapt to osmotic stress caused by salinity. Increased ABA contents in the roots, xylem sap, and leaves were evaluated in two grafting combinations (self-grafted cucumber and cucumber grafted onto pumpkin rootstock). The expression levels of ABA biosynthetic or signaling related genes NCED2 (9-cis-epoxycarotenoid dioxygenase gene 2), ABCG22 (ATP-binding cassette transporter genes 22), PP2C (type-2C protein phosphatases), and SnRK2.1 (sucrose non-fermenting 1-related protein kinases 2) were investigated. Results showed that a root-sourced ABA signal led to decreased stomatal opening and transpiration in the plants grafted onto pumpkin. Furthermore, plants grafted onto pumpkin had increased sensitivity to ABA, compared with self-grafted cucumbers. The inhibition of ABA biosynthesis with fluridon in roots increased the transpiration rate (Tr) and stomatal conductance (Gs) in the leaves. Our study demonstrated that the roots of pumpkin increases the sensitivity of the scion to ABA delivered from the roots to the shoots, and enhances osmotic tolerance under NaCl stress. Such a mechanism can be greatly exploited to benefit vegetable production particularly in semiarid saline regions.
Keywords: abscisic acid; cucumber; grafting; rootstock; salt tolerance; stomata.
Copyright © 2019 Niu, Sun, Nawaz, Sun, Cao, Lu, Huang and Bie.
Figures

Similar articles
-
Grafting cucumber onto luffa improves drought tolerance by increasing ABA biosynthesis and sensitivity.Sci Rep. 2016 Feb 2;6:20212. doi: 10.1038/srep20212. Sci Rep. 2016. PMID: 26832070 Free PMC article.
-
Root respiratory burst oxidase homologue-dependent H2O2 production confers salt tolerance on a grafted cucumber by controlling Na+ exclusion and stomatal closure.J Exp Bot. 2018 Jun 19;69(14):3465-3476. doi: 10.1093/jxb/erx386. J Exp Bot. 2018. PMID: 29145593 Free PMC article.
-
Abscisic Acid-Induced H2O2 Accumulation Enhances Antioxidant Capacity in Pumpkin-Grafted Cucumber Leaves under Ca(NO3)2 Stress.Front Plant Sci. 2016 Sep 30;7:1489. doi: 10.3389/fpls.2016.01489. eCollection 2016. Front Plant Sci. 2016. PMID: 27746808 Free PMC article.
-
Mechanism of Stomatal Closure in Plants Exposed to Drought and Cold Stress.Adv Exp Med Biol. 2018;1081:215-232. doi: 10.1007/978-981-13-1244-1_12. Adv Exp Med Biol. 2018. PMID: 30288712 Review.
-
Function of ABA in Stomatal Defense against Biotic and Drought Stresses.Int J Mol Sci. 2015 Jul 6;16(7):15251-70. doi: 10.3390/ijms160715251. Int J Mol Sci. 2015. PMID: 26154766 Free PMC article. Review.
Cited by
-
Plant Hormone and Inorganic Ion Concentrations in the Xylem Exudate of Grafted Plants Depend on the Scion-Rootstock Combination.Plants (Basel). 2022 Oct 1;11(19):2594. doi: 10.3390/plants11192594. Plants (Basel). 2022. PMID: 36235460 Free PMC article.
-
Low red to far-red light ratio promotes salt tolerance by improving leaf photosynthetic capacity in cucumber.Front Plant Sci. 2023 Jan 6;13:1053780. doi: 10.3389/fpls.2022.1053780. eCollection 2022. Front Plant Sci. 2023. PMID: 36684769 Free PMC article.
-
CmoNAC1 in pumpkin rootstocks improves salt tolerance of grafted cucumbers by binding to the promoters of CmoRBOHD1, CmoNCED6, CmoAKT1;2 and CmoHKT1;1 to regulate H2O2, ABA signaling and K+/Na+ homeostasis.Hortic Res. 2023 Jul 25;10(9):uhad157. doi: 10.1093/hr/uhad157. eCollection 2023 Sep. Hortic Res. 2023. PMID: 37719275 Free PMC article.
-
Research Advancements in Salt Tolerance of Cucurbitaceae: From Salt Response to Molecular Mechanisms.Int J Mol Sci. 2024 Aug 21;25(16):9051. doi: 10.3390/ijms25169051. Int J Mol Sci. 2024. PMID: 39201741 Free PMC article. Review.
-
Multiple Stressors in Vegetable Production: Insights for Trait-Based Crop Improvement in Cucurbits.Front Plant Sci. 2022 May 3;13:861637. doi: 10.3389/fpls.2022.861637. eCollection 2022. Front Plant Sci. 2022. PMID: 35592574 Free PMC article. Review.
References
-
- Bassil E., Tajima H., Liang Y. C., Ohto M. A., Ushijima K., Nakano R., et al. (2011). The Arabidopsis Na+/H+ antiporters NHX1 and NHX2 control vacuolar pH and K+ homeostasis to regulate growth, flower development, and reproduction. Plant Cell 23 (9), 3482–3497. 10.1105/tpc.111.089581 - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous

