Mutagenesis in Rice: The Basis for Breeding a New Super Plant
- PMID: 31781133
- PMCID: PMC6857675
- DOI: 10.3389/fpls.2019.01326
Mutagenesis in Rice: The Basis for Breeding a New Super Plant
Abstract
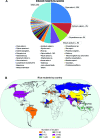
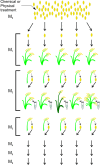
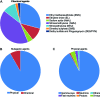
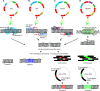
The high selection pressure applied in rice breeding since its domestication thousands of years ago has caused a narrowing in its genetic variability. Obtaining new rice cultivars therefore becomes a major challenge for breeders and developing strategies to increase the genetic variability has demanded the attention of several research groups. Understanding mutations and their applications have paved the way for advances in the elucidation of a genetic, physiological, and biochemical basis of rice traits. Creating variability through mutations has therefore grown to be among the most important tools to improve rice. The small genome size of rice has enabled a faster release of higher quality sequence drafts as compared to other crops. The move from structural to functional genomics is possible due to an array of mutant databases, highlighting mutagenesis as an important player in this progress. Furthermore, due to the synteny among the Poaceae, other grasses can also benefit from these findings. Successful gene modifications have been obtained by random and targeted mutations. Furthermore, following mutation induction pathways, techniques have been applied to identify mutations and the molecular control of DNA damage repair mechanisms in the rice genome. This review highlights findings in generating rice genome resources showing strategies applied for variability increasing, detection and genetic mechanisms of DNA damage repair.
Keywords: DNA repair; Oryza sativa L; functional genomics; genetic variability; mutagenesis; mutation detection; random mutations; targeted mutations.
Copyright © 2019 Viana, Pegoraro, Busanello and Costa de Oliveira.
Figures

Similar articles
-
The Genomics of Oryza Species Provides Insights into Rice Domestication and Heterosis.Annu Rev Plant Biol. 2019 Apr 29;70:639-665. doi: 10.1146/annurev-arplant-050718-100320. Annu Rev Plant Biol. 2019. PMID: 31035826 Review.
-
CRISPR/Cas9 Guided Mutagenesis of Grain Size 3 Confers Increased Rice (Oryza sativa L.) Grain Length by Regulating Cysteine Proteinase Inhibitor and Ubiquitin-Related Proteins.Int J Mol Sci. 2021 Mar 22;22(6):3225. doi: 10.3390/ijms22063225. Int J Mol Sci. 2021. PMID: 33810044 Free PMC article.
-
Applications of the CRISPR/Cas9 System for Rice Grain Quality Improvement: Perspectives and Opportunities.Int J Mol Sci. 2019 Feb 19;20(4):888. doi: 10.3390/ijms20040888. Int J Mol Sci. 2019. PMID: 30791357 Free PMC article. Review.
-
Rice breeding in the post-genomics era: from concept to practice.Curr Opin Plant Biol. 2013 May;16(2):261-9. doi: 10.1016/j.pbi.2013.03.008. Epub 2013 Apr 6. Curr Opin Plant Biol. 2013. PMID: 23571011 Review.
-
[Major domestication traits in Asian rice].Yi Chuan. 2012 Nov;34(11):1379-89. doi: 10.3724/sp.j.1005.2012.01379. Yi Chuan. 2012. PMID: 23208135 Review. Chinese.
Cited by
-
Ethyl Methane Sulfonate and Sodium Azide-Mediated Chemical and X-ray-Mediated Physical Mutagenesis Positively Regulate Peroxidase 1 Gene Activity and Biosynthesis of Antineoplastic Vinblastine in Catharanthus roseus.Plants (Basel). 2022 Oct 28;11(21):2885. doi: 10.3390/plants11212885. Plants (Basel). 2022. PMID: 36365340 Free PMC article.
-
Exploiting Genic Male Sterility in Rice: From Molecular Dissection to Breeding Applications.Front Plant Sci. 2021 Mar 2;12:629314. doi: 10.3389/fpls.2021.629314. eCollection 2021. Front Plant Sci. 2021. PMID: 33763090 Free PMC article. Review.
-
Organ-specific expression of genes involved in iron homeostasis in wheat mutant lines with increased grain iron and zinc content.PeerJ. 2022 Jun 10;10:e13515. doi: 10.7717/peerj.13515. eCollection 2022. PeerJ. 2022. PMID: 35707120 Free PMC article.
-
Characterization of Cell Wall Compositions of Sodium Azide-Induced Brittle Mutant Lines in IR64 Variety and Its Potential Application.Plants (Basel). 2024 Nov 25;13(23):3303. doi: 10.3390/plants13233303. Plants (Basel). 2024. PMID: 39683096 Free PMC article.
-
A new method for mutation inducing in rice by using DC electrophoresis bath and its mutagenic effects.Sci Rep. 2023 Apr 25;13(1):6707. doi: 10.1038/s41598-023-33742-7. Sci Rep. 2023. PMID: 37185291 Free PMC article.
References
-
- Akter M. B., Piao R., Kim B., Lee Y., Koh E., Koh H.-J. (2014). Fine mapping and candidate gene analysis of a new mutant gene for panicle apical abortion in rice. Euphytica 197, 387–398. 10.1007/s10681-014-1074-8 - DOI
-
- Almadanim M. C., Alexandre B. M., Rosa M. T. G., Sapeta H., Leitão A. E., Ramalho J. C., et al. (2017). Rice calcium-dependent protein kinase OsCPK17 targets plasma membrane intrinsic protein and sucrose-phosphate synthase and is required for a proper cold stress response. Plant. Cell Environ. 40, 1197–1213. 10.1111/pce.12916 - DOI - PubMed
Publication types
LinkOut - more resources
Full Text Sources

