Development of Parasitic Organs of a Stem Holoparasitic Plant in Genus Cuscuta
- PMID: 31781146
- PMCID: PMC6861301
- DOI: 10.3389/fpls.2019.01435
Development of Parasitic Organs of a Stem Holoparasitic Plant in Genus Cuscuta
Abstract
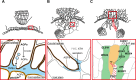
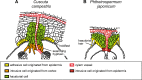
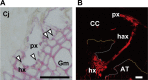
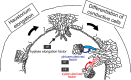
Parasitic plants infect a broad range of plant species including economically important crops. They survive by absorbing water, minerals, and photosynthates from their hosts. To support their way of life, parasitic plants generally establish parasitic organs that allow them to attach to their hosts and to efficiently absorb substances from the vascular system of the host. Here, we summarize the recent progress in understanding the mechanisms underlying the formation of these parasitic organs, focusing on the process depicted in the stem holoparasitic genus, Cuscuta. An attachment structure called "holdfast" on the stem surface is induced by the light and contact stimuli. Concomitantly with holdfast formation, development of an intrusive structure called haustorium initiates in the inner cortex of the Cuscuta stem, and it elongates through apoplastic space of the host tissue. When haustoria reaches to host vascular tissues, they begin to form vascular conductive elements to connect vascular tissue of Cuscuta stem to those of host. Recent studies have shown parasite-host interaction in the interfacial cell wall, and regulation of development of these parasitic structures in molecular level. We also briefly summarize the role of host receptor in the control of compatibility between Cuscuta and hosts, on which occurrence of attachment structure depends, and the role of plant-to-plant transfer of long-distance signals after the establishment of conductive structure.
Keywords: Cuscuta; attachment cells; conductive cells; haustorium; host factors; intrusive cells; parasitic organs; parasitic plants.
Copyright © 2019 Shimizu and Aoki.
Figures

References
Publication types
LinkOut - more resources
Full Text Sources

