miR-129-5p Inhibits Adipogenesis through Autophagy and May Be a Potential Biomarker for Obesity
- PMID: 31781210
- PMCID: PMC6875017
- DOI: 10.1155/2019/5069578
miR-129-5p Inhibits Adipogenesis through Autophagy and May Be a Potential Biomarker for Obesity
Abstract
Introduction: Obesity has an unclear pathogenesis. MicroRNAs (miRNAs) may function as biologically active molecules for obesity through regulating adipocyte differentiation. This study aimed to identify how miR-129-5p (a specific miRNA) regulates adipogenesis in vitro and explore its possible role in the pathogenesis of obesity in humans.
Materials and methods: The miR-129-5p expression was detected in obese mouse models. The effect of miR-129-5p on adipocyte differentiation was observed, and the adipose markers were analyzed. Bioinformatics and dual-luciferase reporter assay were applied to predict and confirm the target genes of miR-129-5p. The human serum samples were detected and analyzed.
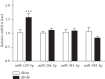
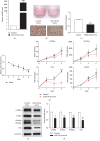
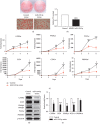
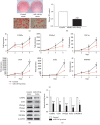
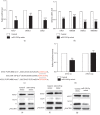
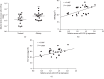
Results: miR-129-5p is highly expressed in adipose tissues of db/db mice. Gain- and loss-of-function studies show that miR-129-5p could significantly inhibit adipocyte differentiation and white adipocyte browning in vitro and decreases the level of specific markers, such as FABP4, UCP1, and PPARγ, in mature white and brown adipocytes. miR-129-5p directly targets ATG7 which is predicted with bioinformatics and confirmed by dual-luciferase reporter assay. Serum miR-129-5p level was evidently elevated in patients with simple obesity (p < 0.01) and correlates with obesity indices, including BMI (r = 0.407, p < 0.029) and fat percentage (r = 0.394, p < 0.038).
Conclusion: miR-129-5p might target on the ATG7-related autophagy signaling network that regulates white and brown adipogenesis. Importantly, the aforementioned results suggest serum miR-129-5p might be a potential biomarker and therapeutic target for obesity.
Copyright © 2019 Xue Fu et al.
Conflict of interest statement
The authors declare that they have no conflicts of interest.
Figures
Similar articles
-
miR-669a-5p promotes adipogenic differentiation and induces browning in preadipocytes.Adipocyte. 2022 Dec;11(1):120-132. doi: 10.1080/21623945.2022.2030570. Adipocyte. 2022. PMID: 35094659 Free PMC article.
-
MiR-9-5p promotes rabbit preadipocyte differentiation by suppressing leptin gene expression.Lipids Health Dis. 2020 Jun 5;19(1):126. doi: 10.1186/s12944-020-01294-8. Lipids Health Dis. 2020. PMID: 32503618 Free PMC article.
-
Integrative analysis of miRNA and mRNA profiles reveals that gga-miR-106-5p inhibits adipogenesis by targeting the KLF15 gene in chickens.J Anim Sci Biotechnol. 2022 Jul 6;13(1):81. doi: 10.1186/s40104-022-00727-x. J Anim Sci Biotechnol. 2022. PMID: 35791010 Free PMC article.
-
Adipogenic miRNA and meta-signature miRNAs involved in human adipocyte differentiation and obesity.Oncotarget. 2016 Jun 28;7(26):40830-40845. doi: 10.18632/oncotarget.8518. Oncotarget. 2016. PMID: 27049726 Free PMC article. Review.
-
MicroRNAs as Epigenetic Regulators of Obesity.Adv Exp Med Biol. 2024;1460:595-627. doi: 10.1007/978-3-031-63657-8_20. Adv Exp Med Biol. 2024. PMID: 39287866 Review.
Cited by
-
The downregulation of miR-129-5p relieves the inflammatory response in acute respiratory distress syndrome by regulating PPARγ-mediated autophagy.Ann Transl Med. 2022 Mar;10(6):345. doi: 10.21037/atm-22-979. Ann Transl Med. 2022. PMID: 35433953 Free PMC article.
-
Lipophagy: Molecular Mechanisms and Implications in Metabolic Disorders.Mol Cells. 2020 Aug 31;43(8):686-693. doi: 10.14348/molcells.2020.0046. Mol Cells. 2020. PMID: 32624503 Free PMC article. Review.
-
miR-129 Regulates Yak Intramuscular Preadipocyte Proliferation and Differentiation through the PI3K/AKT Pathway.Int J Mol Sci. 2024 Jan 3;25(1):632. doi: 10.3390/ijms25010632. Int J Mol Sci. 2024. PMID: 38203803 Free PMC article.
-
Dysmetabolic adipose tissue in obesity: morphological and functional characteristics of adipose stem cells and mature adipocytes in healthy and unhealthy obese subjects.J Endocrinol Invest. 2021 May;44(5):921-941. doi: 10.1007/s40618-020-01446-8. Epub 2020 Nov 3. J Endocrinol Invest. 2021. PMID: 33145726 Review.
-
The miRNomics of antiretroviral therapy-induced obesity.Funct Integr Genomics. 2025 Apr 5;25(1):81. doi: 10.1007/s10142-025-01585-2. Funct Integr Genomics. 2025. PMID: 40186666 Free PMC article. Review.
References
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

